Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1450
Revised: November 24, 2002
Accepted: November 28, 2002
Published online: July 15, 2003
AIM: Now many countries have developed cancer therapy with heavy ions, especially in GSI (Gesellschaft fürSchwerionenforschung mbH, Darmstadt, Germany), remarkable results have obtained, but due to the complexity of particle track structure, the basic theory still needs further researching. In this paper, the genotoxic effects of heavy ions irradiation on SMMC-7721 cells were measured using the single cell gel electrophoresis (comet assay). The information about the DNA damage made by other radiations such as X-ray, γ-ray, UV and fast neutron irradiation is very plentiful, while little work have been done on the heavy ions so far. Hereby we tried to detect the reaction of liver cancer cells to heavy ion using comet assay, meanwhile to establish a database for clinic therapy of cancer with the heavy ions.
METHODS: The human hepatoma cells were chosen as the test cell line irradiated by 80Mev/u 20Ne10+ on HIRFL (China), the radiation-doses were 0, 0.5, 1, 2, 4 and 8 Gy, and then comet assay was used immediately to detect the DNA damages, 100-150 cells per dose-sample (30-50 cells were randomly observed at constant depth of the gel). The tail length and the quantity of the cells with the tail were put down. EXCEL was used for statistical analysis.
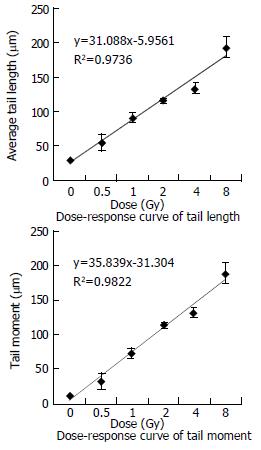
RESULTS: We obtained clear images by comet assay and found that SMMC-7721 cells were all damaged apparently from the dose 0.5 Gy to 8 Gy (t-test: P < 0.001, vs control). The tail length and tail moment increased as the doses increased, and the number of cells with tails increased with increasing doses. When doses were higher than 2 Gy, nearly 100% cells were damaged. Furthermore, both tail length and tail moment, showed linear equation.
CONCLUSION: From the clear comet assay images, our experiment proves comet assay can be used to measure DNA damages by heavy ions. Meanwhile DNA damages have a positive correlation with the dose changes of heavy ions and SMMC-7721 cells have a great radiosensitivity to 20Ne10+. Different reactions to the change of doses indicate that comet assay is a useful tool to detect DNA damage induced by heavy ions.
- Citation: Qiu LM, Li WJ, Pang XY, Gao QX, Feng Y, Zhou LB, Zhang GH. Observation of DNA damage of human hepatoma cells irradiated by heavy ions using comet assay. World J Gastroenterol 2003; 9(7): 1450-1454
- URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1450
During the past few decades, radiation research has developed into specialized sub-disciplines, from basic physics and chemistry to tumor biology and experimental radiation therapy[1]. Although the radiobiological effects are extensively investigated for X-ray and γ-ray, little work has been directed towards heavy ion beams. With the exploration of the outer space, the research of high linear energy transfer (LET) has attracted more and more attention. Since heavy ions were first applied in the mid-1970s to cure cancer at Bevalac of Lawrence Berkeley Laboratory (LBL), United States, promising results have been reported when compared with the conventional radiotherapy for soft tissue sarcoma, bone sarcoma and prostate cancer[2]. Now scientists in many countries (GSI in Germany, HIMAC in Japan[25,26], HIRFL in China) have designed accelerators to deliver beams of ions for the treatment and started basic researches of cancer therapy with heavy ions such as carbon, neon, oxygen and argon.
The aim of our present study was to investigate DNA damages induced by heavy ions by comet assay. The theoretical value was then compared to responses to external X-ray or γ-ray and other irradiations, so that we could establish the data base for clinical therapy.
Comet assay, the alkaline version in particular, has become a very popular method for the analysis of DNA damage caused by various chemical and physical agents because of its simplicity and rapidity[4-10]. DNA damages consisted of DNA strand breakage, alkali-labile sites and incomplete excision repair sites[11]. Although the direct DNA-breaking capacity can be estimated by alkaline elution, nick translation and alkaline single cell gel electrophoresis (SCGE), SCGE has been shown to be more sensitive than the former two. It had been proved that the sensitivity of SCGE is significantly higher than that of cytokinesis-blocked micronucleus (CBMN) test[12]. The most important point is that comet assay is an electrophoretic technique, which allows measurements of DNA damages as well as DNA repair rates on an individual cell. Therefore its contribution to DNA damages by irradiating cells with heavy ions at once or after a while can be reflected as initial damages or residual DNA damages, if time is allowed for enzymatic repairs of initial DNA strand break. In our lab, we focused on the radiobiological effects of heavyions on tumor cells. Previous experiments were mainly on cell survival measurements and could not explain the underlying radiosensitization mechanism at molecular level[2]. To verify the radiobiological effects of heavy ions on cellular DNA, SCGE also called comet assay was used to directly measure DNA damages in cells.
Human hepatoma cells SMMC-7721, purchased from Second Military Medical University in Shanghai, were cultivated in RMIP-1640 medium (Gibco product) supplemented with 15% calf serum in a standard incubator at 37 °C. One passage of cells every 2-3 d and change of the medium everyday were performed, to ensure the cells growth in good conditions. Two days before the irradiation, the cells were shifted to Φ 35 mm petri-dishes, each had 2 mL cell suspension, and the density of the cells was 5 × 104 cells/ml. Each dose had 5 petri-dishes. Before irradiation, cells in each petri-dish were examined under the inverted light microscope to select materials good in growth and even in density, and medium 1640 in petri-dishes was removed, only Dulbecc's phosphate -buffered saline medium (PBS) was left to keep the moisture of the cells when irradiated.
Irradiation was performed using 20Ne10+ with energy of 80 Mev/u and intensity of 0.00136nA (2.1 × 106p/s). The cells were irradiated at the doses of 0, 0.5, 1, 2, 4, 8 Gy. The doses of cells were measured by an air ionization chamber.
As soon as irradiation ended, the cells were washed and collected, the final concentration of cells was adjusted to (5-10) 106 by adding Dulbecc's phosphate-buffered saline medium (PBS) to the single cell suspension.
The alkaline version of comet assay was carried out based on the work of Ostling and Johansson with some minor modifications as followings: On the day of electrophoresis, an aliquot of 10 μL freshly prepared suspension of cells was mixed with 30 μL 0.5% low- melting-agarose in Dulbecc's PBS (pH7.4). The mixture was layered on top of an ordinary microscope slide precoated with 0.5% normal-melting -agorose, which was allowed to dry at room temperature protected from dust and other particles. After low-melting-agarose was solidified in a refrigerator for 10 min, the coverslip was carefully removed and the slide was gently immersed in a freshly prepared lysing solution (2.5 M NaCl, 10 mM Tris, 1% sodium lauryl sarcosinate, 100 mM Na2EDTA, with 1% Triton-100 and 10% DMSO added just before use). From this moment until the end of neutralization, all steps needed to avoid the sunlight.
After lysis for 1-1.5 h, the microscopy slides were transferred to electrophoresis session, 18 microscopy slides from 6 samples (3 slides/each sample) were randomly placed in a electrophoretic unit.
After 20-30 min of DNA unwinding in electrophoresis buffer (1 mM EDTA-Na2, 300 mM NaOH, pH > 13), single cell gel electrophoresis was performed in the same buffer (20 min, 20 V, 300 nA). After electrophoresis the slides were neutralized with 0.4 M Tris buffer (pH7.5).
The microscopy slides were stained with ethidium bromide in water (40 μg/μL, 50 μL/slide). After application of a coverslip was removed, each slide was examined at 10 × 20 magnification in a fluorescence microscope (excitation filter: 400nm, barrier filter: 590nm). 100-150 cells per dose-sample (30-50 cell were randomly observed at constant depth of the gel, avoiding the edges of the gel on each of three replicate slides). The tail length and the quantity of the cells with the tail were put down, at the same time, photos were taken with 135# black and white film (ISO 400). Then analysis was done using EXCEL.
The comet assay is attractive for many reasons. Apart from being a quick, simple, sensitive, reliable and fairly inexpensive way of measuring, it also produces appealing images.
There are two explanations about what the comet tails consist of. One is that it is a fragment DNA, the other is that the length of such a fragment is about 1 mm, but the length of the tail of a comet is a few percentages of it[13-16], as to our experiment the longest mean length of tail was no more than 200 mm. Nuclear DNA is not a tangle of string, even after treatment with detergents and a strong salt solution, as in the SCGE procedure, the nuclear (or nucleoid) had a structure, the DNA was organized as loops, which retained the super coils that were contained in the nucleosome. Cook et al[17] deduced the presence of supercoiled loops and then they observed that, when DNA was broken by irradiation, supercoiling was relaxed and loops spilled out into a 'halo' around the nucleoid. By analogy, it is assumed that the Comet tail is made up of relaxed loops, and that the number of loops in the tail indicates the number of DNA breaks.
The alkaline comet assay can detect DNA breaks including single and double DNA strand breaks, and AP sites, which are alkali-labile and probably converted to breaks while DNA is in the electrophoretic solution at high pH[14-16]. The present comet assay is generally practiced including incubation of DNA at high pH before and during electrophoresis, different from the original work of Ostling and Johansson who employed near-neutral pH. Collins AR[13] proved that both the neutral and alkaline methods could detect low levels of DNA damage, however, the breaks by higher levels of damage were more clearly resolved from the head under alkaline conditions[18]. Thus in our experiment, the alkaline version was used.
Furthermore, breaks will be transiently present when cells repair lesions via base excision or nucleotide excision so that a high level of breaks in the Comet assay may indicate either severe damage or efficient repair[13]. In fact, much useful information can be obtained by exploiting cellular repair to produce DNA breaks and thus to reveal or amplify the effect of radiation that otherwise may not show positive effects by the comet assay. This will be discussed in our later work on the repair effects of heavy ions.
The SCGE test or comet assay is a straightforward visual method for the detection of DNA damage of cells in interphase. This technique is especially sensitive in detecting DNA single-strand breaks, alkali-labile damage and excision repair sites in individual cells[13-16]. The Comet assay has been widely applied in the following fields: radiation biology[5-6,9-12,19], excisable DNA damage, DNA cross-links[20], oxidative damage[21,22], genetic toxicology and apoptosis[23,24].
Ionizing radiation is a ubiquitous environmental agent. Its physiochemical interaction with cellular DNA produces a variety of primary lesions, such as single-strand breaks (SSB), double-strand breaks (DSB), DNA-DNA and DNA-protein crosslinks, and damage to purine and pyrimidine bases. And using ionizing radiation may avoid complications of drug metabolism, intracellar distribution, membrane permeability and drug efflux. Although the technique of SCGE is very sensitive to ionizing radiation, information about DNA damage made by other radiations such as X-ray[10,12], γ-ray[8,9], UV[9] and fast neutron[19] irradiation is very plentiful, little work has been done on heavy ions so far.
Microdosimetric considerations suggest that, in a given type of radiation, the yield DNA damage must be proportional to dose, so that besides the influence of changing repair efficiency, the magnitude of the dose might not be expected to be critical in comparison of the results. Heavy ion is a kind of high LET (Linear Energy Transfer) irradiation, as emphasized long ago by Lea, high-LET radiation could, through the increased frequency of DSB in close proximity, cause interactive damages and misrepair[27-28]. We anticipated that heavy ions probably had strong effects on the cellular DNA, but we wonder if it can make the linear equation after irradiation by heavy ions.
In our experiment, the data for DNA damages induced by heavy ions at the doses of 0-8 Gy are presented in Table 1. The dose-response curves for tail length and tail moment (the fraction of DNA in the tail multiplied by tail length) are shown in Figure 2. We could see tail length and tail moment showed linear equation. It proved that SMMC-7721 cells have high radiosensitivity to heavy ions and comet assay is very useful to detect DNA damages induced by heavy ions.
| Dose Gy | Tail length mean ± S.E. μm | Tail DNA mean ± S.E % | Tail moment mean ± S.E. | t-test P |
| 0 | 29.44 ± 1.46 | 38.50 ± 3.50 | 11.53 ± 1.45 | |
| 0.5 | 54.18 ± 11.74 | 59.21 ± 9.21 | 33.02 ± 11.80 | 2.06227E-07 |
| 1 | 90.16 ± 6.66 | 85.54 ± 2.21 | 76.96 ± 3.44 | 8.7291E-21 |
| 2 | 115.09 ± 3.26 | 100.00 ± 0.00 | 115.09 ± 3.25 | 6.97944E-31 |
| 4 | 134.17 ± 8.18 | 98.86 ± 1.07 | 134.17 ± 8.10 | 4.48617E-68 |
| 8 | 194.08 ± 15.58 | 100.00 ± 0.00 | 194.08 ± 15.58 | 6.4087E-104 |
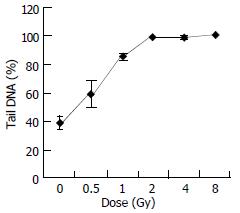
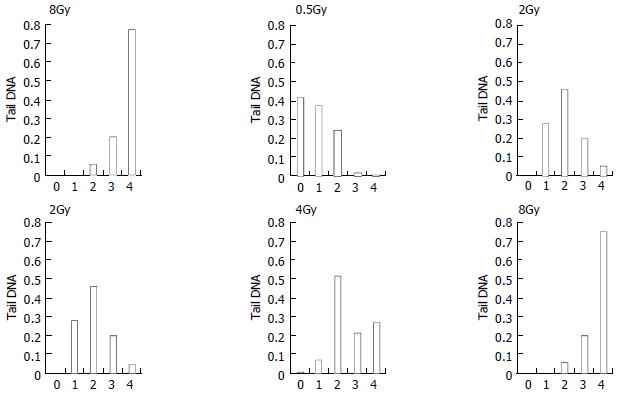
Figure 3 shows the change of tail DNA as the dose increased. It was found that almost 100% cells were damaged when the dose reached at 2 Gy. But the details were unknown about how badly DNA was damaged when the dose was higher than 2 Gy. To completely evaluate DNA damages by different doses, comets of every dose-sample were sorted visually into classes 0-4, representing increasing amount of damage. Figure 4, result shows that with increase of the dose, slighter damage of DNA tail (class 0-2) converted to more severe change of DNA (DNA migrated from the head to form longer and longer tail).
We should pay more attention to the dose of 2 Gy, which is the conventional choice of γ-ray radiotherapy. At the dose of 2 Gy, 100% cells were damaged, with different grade of DNA damage (classes 1-4). Among them nearly 25% cells were badly damaged. It is known that a central phenomenon in radiobiology is the efficiency of densely ionizing radiation for cellular effects. As in our experiment, chromosomal aberrations or cell killing occurred on these badly damaged cells[27-30,33-35]. Additionally, we noticed that nearly 80% cells were most severely damaged (class 4) at the dose of 8 Gy. It showed that 8 Gy might be or near the highest dose that the cells could withstand.
One thing to be mentioned here is that the relative biological effectiveness (RBE) tends to increase with linear energy transfer (LET). For very heavy ions with LET in excess of about 100-200 kev/mm, a more complex dependence on particle track structure emerges[31,32]. Therefore, the study on particle track structure is very important for further research.
Edited by Zhao P, Zhu LH and Wang XL
| 1. | Dörr W, Dörschel B, Sprinz H. Report on the third annual meeting of the Society for Biological Radiation Research, GBS '99. Radiat Environ Biophys. 2000;39:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 2. | Li WJ, Gao QX, Zhou GM, Wei ZQ. Micronuclei and cell survival in human liver cancer cells irradiated by 25MeV/u (40)Ar14(+). World J Gastroenterol. 1999;5:365-368. [PubMed] |
| 3. | Zhou GM, Chen WQ, Gao QX, Li WJ, Li Q, Wei ZQ. Biological effects of hepatoma cells irradiated by 25MeV/u(40)Ar(14+). World J Gastroenterol. 1998;4:271-272. [PubMed] |
| 4. | Wojewódzka M, Kruszewski M, Iwaneñko T, Collins AR, Szumiel I. Application of the comet assay for monitoring DNA damage in workers exposed to chronic low-dose irradiation. I. Strand breakage. Mutat Res. 1998;416:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Kruszewski M, Wojewódzka M, Iwanenko T, Collins AR, Szumiel I. Application of the comet assay for monitoring DNA damage in workers exposed to chronic low-dose irradiation. II. Base damage. Mutat Res. 1998;416:37-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Müller WU, Bauch T, Stüben G, Sack H, Streffer C. Radiation sensitivity of lymphocytes from healthy individuals and cancer patients as measured by the comet assay. Radiat Environ Biophys. 2001;40:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Wojewódzka M, Kruszewski M, Iwanenko T, Collins AR, Szumiel I. Lack of adverse effect of smoking habit on DNA strand breakage and base damage, as revealed by the alkaline comet assay. Mutat Res. 1999;440:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Mayer C, Popanda O, Zelezny O, von Brevern MC, Bach A, Bartsch H, Schmezer P. DNA repair capacity after gamma-irradiation and expression profiles of DNA repair genes in resting and proliferating human peripheral blood lymphocytes. DNA Repair (. Amst). 2002;1:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Mohankumar MN, Paul SF, Venkatachalam P, Jeevanram RK. Influence of in vitro low-level gamma-radiation on the UV-induced DNA repair capacity of human lymphocytes--analysed by unscheduled DNA synthesis (UDS) and comet assay. Radiat Environ Biophys. 1998;37:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Koppen G, Angelis KJ. Repair of X-ray induced DNA damage measured by the comet assay in roots of Vicia faba. Environ Mol Mutagen. 1998;32:281-285. [PubMed] [DOI] [Full Text] |
| 11. | Cebulska-Wasilewska A, Nowak D, Niedźwiedź W, Anderson D. Correlations between DNA and cytogenetic damage induced after chemical treatment and radiation. Mutat Res. 1998;421:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | He JL, Chen WL, Jin LF, Jin HY. Comparative evaluation of the in vitro micronucleus test and the comet assay for the detection of genotoxic effects of X-ray radiation. Mutat Res. 2000;469:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Collins AR, Dobson VL, Dusinská M, Kennedy G, Stĕtina R. The comet assay: what can it really tell us. Mutat Res. 1997;375:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 439] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | McNamee JP, McLean JR, Ferrarotto CL, Bellier PV. Comet assay: rapid processing of multiple samples. Mutat Res. 2000;466:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Godard T, Gauduchon P, Debout C. A first step in visual identification of different cell populations by a modified alkaline comet assay. Mutat Res. 2002;520:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Ruiz de Almodóvar JM, Guirado D, Isabel Núñez M, López E, Guerrero R, Valenzuela MT, Villalobos M, del Moral R. Individualization of radiotherapy in breast cancer patients: possible usefulness of a DNA damage assay to measure normal cell radiosensitivity. Radiother Oncol. 2002;62:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Cook PR, Brazell IA, Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci. 1976;22:303-324. [PubMed] |
| 18. | Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V. Recommendations for conducting the in vivo alkaline Comet assay. 4th International Comet Assay Workshop. Mutagenesis. 2003;18:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 737] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 19. | Gajendiran N, Tanaka K, Kamada N. Comet assay to sense neutron 'fingerprint'. Mutat Res. 2000;452:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Merk O, Reiser K, Speit G. Analysis of chromate-induced DNA-protein crosslinks with the comet assay. Mutat Res. 2000;471:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Guetens G, De Boeck G, Highley M, van Oosterom AT, de Bruijn EA. Oxidative DNA damage: biological significance and methods of analysis. Crit Rev Clin Lab Sci. 2002;39:331-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Godard T, Deslandes E, Sichel F, Poul JM, Gauduchon P. Detection of topoisomerase inhibitor-induced DNA strand breaks and apoptosis by the alkaline comet assay. Mutat Res. 2002;520:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Heng Z, Li R, Zhang Z. [Distinguishing apoptotic cells from DNA strand-broken cells by comet assay]. Wei Sheng Yan Jiu. 2001;30:149-151. [PubMed] |
| 24. | Roser S, Pool-Zobel BL, Rechkemmer G. Contribution of apoptosis to responses in the comet assay. Mutat Res. 2001;497:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Ando K. High LET radiobiology at NIRS--current status and future plan. Phys Med. 2001;17 Suppl 1:292-295. [PubMed] |
| 26. | Imamura M, Murata T, Akagi K, Tanaka Y, Imamura M, Inoue K, Mizuma N, Kobayashi Y, Watanabe H, Hachiya M. Relationship between LET and RBE values for Escherichia coli determined using carbon ion beams from the TIARA cyclotron and HIMAC synchrotron. J Gen Appl Microbiol. 1997;43:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Sutherland BM, Bennett PV, Schenk H, Sidorkina O, Laval J, Trunk J, Monteleone D, Sutherland J. Clustered DNA damages induced by high and low LET radiation, including heavy ions. Phys Med. 2001;17 Suppl 1:202-204. [PubMed] |
| 28. | Goodwin EH, Blakely EA. Heavy ion-induced chromosomal damage and repair. Adv Space Res. 1992;12:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Blakely EA. New measurements for hadrontherapy and space radiation: biology. Phys Med. 2001;17 Suppl 1:50-58. [PubMed] |
| 30. | Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol Biol Phys. 2000;48:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Katz R, Cucinotta FA. Tracks to therapy. Radiat Meas. 1999;31:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Kraxenberger F, Weber KJ, Friedl AA, Eckardt-Schupp F, Flentje M, Quicken P, Kellerer AM. DNA double-strand breaks in mammalian cells exposed to gamma-rays and very heavy ions. Fragment-size distributions determined by pulsed-field gel electrophoresis. Radiat Environ Biophys. 1998;37:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Edwards AA. RBE of radiations in space and the implications for space travel. Phys Med. 2001;17 Suppl 1:147-152. [PubMed] |