Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.978
Revised: October 29, 2002
Accepted: November 7, 2002
Published online: May 15, 2003
AIM: To characterize the host response to hepatitis B virus (HBV) infection in human hepatocytes transplanted into immunocompetent rodent rats tolerized by, and transplanted with primary human hepatocytes.
METHODS: One week after the transplantation, rats were inoculated with HBV, and viral gene expression, replication, and host response was monitored.
RESULTS: HBV DNA was detectable in serum for at least 60 days. HBsAg levels rose steadily for 3 weeks post-inoculation and then plateaued at a level of about 0.6 pg/ml. HBV RNA was also found in liver at levels that remained constant through the time course. Immunofluorescence revealed clusters of hepatocytes that stained positive for HBcAg. The presence of HBV covalently closed circular DNA (cccDNA) in liver was demonstrated using nuclease digestion of single-stranded DNA followed by PCR. Serum ALT levels rose and reached a peak level of 180 IU/L on day 18, but remained elevated for 60 days. Histology revealed a progressive predominantly mononuclear lobular hepatitis.
CONCLUSION: These data indicate that human hepatocytes transplanted into rats rendered tolerant to these cells, when infected by HBV, results in biochemical as well as histological evidence of hepatitis that accompanies viral gene expression, and DNA replication.
- Citation: Wu CH, Ouyang EC, Walton C, Promrat K, Forouhar F, Wu GY. Hepatitis B virus infection of transplanted human hepatocytes causes a biochemical and histological hepatitis in immunocompetent rats. World J Gastroenterol 2003; 9(5): 978-983
- URL: https://www.wjgnet.com/1007-9327/full/v9/i5/978.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.978
It has been shown recently that primary human hepatocytes can be transplanted into rats and infected with human hepatitis B virus[1]. This was accomplished by rendering normal rats tolerant to human hepatocytes by intrafetal injection of primary hepatocytes. The rats were then transplanted with the same batches of cells, and then infected by inoculation of those animals with purified HBV. This resulted in production of HBV antigens. The current studies were performed to evaluate the effects of this infection on the host liver.
Pregnant Sprague-Dawley rats, 250 to 300 g of body weight (Charles River Co., Inc., Wilmington, MA) were maintained on 12 hour light-dark cycles, and fed ad lib with standard rat chow in the Center for Laboratory Animal Care at the University of Connecticut Health Center. All animal procedures were approved by Institutional Animal Care and Use Committee and conformed to USDA and NIH animal usage guidelines.
Human hepatocytes were obtained from Clonetics Corp (Walkersville, MD). HepG2 2.2.15[2] cells, a transformed human liver cell line that constitutively produces infectious HBV, were grown in Dulbecco Modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and antibiotics.
Rats were rendered tolerant to human hepatocytes by intrafetal injection of those cells as described previously[1]. In brief, at 15 to 17 days of gestation, groups of pregnant rats were anesthetized by intramuscular injections of ketamine (40 mg/kg body weight) and xylazine (5 mg/kg body weight) as described previously[3]. Laparatomies were performed under sterile conditions; gravid uteri were exposed, and transilluminated by a high intensity lamp (Fiber-lite MI-150, Dolan-Jenner Industries, Lawrence, MA). Human hepatocytes, 1 × 105 cells in 10 μl PBS, were injected through the uterine wall into the peritoneal cavities of rat fetuses using a sterile 200 μl Hamilton syringe with a 28 gauge beveled point needle (Hamilton Inc., Reno, NV).
Within 24 hours of birth, newborn rats were placed on ice for 2-5 min, and 2 × 106 human hepatocytes in 200 μl PBS were injected into the spleen by sterile Hamilton syringe.
Peripheral blood samples were drawn from tail veins, spun, and serum stored at -20 °C. Liver samples were collected either by sacrificing animals or by performing partial hepatectomies. Samples were fresh frozen in liquid nitrogen, and stored at -80 °C.
One week following human hepatocyte transplantation, tolerized rats were inoculated with 105 HBV particles in 100 μl TEN (0.02 M Tris-HCl, pH7.5, 1 mM EDTA, 0.15 M NaCl) buffer by intrasplenic injection. Controls consisted of tolerized neonatal rats from the same litter that did not receive human hepatocyte transplantation, but were also inoculated identically with HBV, as well as tolerized rats from the same litter that transplanted with human hepatocytes, but no HBV, and tolerized rats from the same litter with saline injection only.
Levels of HBsAg in rat serum were measured as a function of time after inoculation using an enzyme-linked immunoassay kit for HBV surface antigen (Abbott Labs) according to the manufacturer’s protocol. Antigen was quantitated using a spectrophotometer at 492 nm. Assays were done in triplicate, and the results expressed as means ± SD in units of pg/ml serum.
To detect circulating virus, HBV DNA was extracted from 30 μl of rat serum, incubated with 100 μg/ml proteinase K in 0.05 M Tris-HCl, pH8.0, 0.1 M EDTA and 0.5% SDS, overnig ht at 55 °C. DNA was purified bypheno l-chloroform extraction[4]. An antisense primer 5’-ATCTTCTGCGACGCGGCGATGGAGATC-3’ and a sense primer 5’-CTCTGCTGGGGGGAATTGATGACTCTAGC-3’ were used to generate an expected 355 bp fragment of adw HBV genome spanning nt 2079-2434. One third of total cDNA was mixed with 100 pmol of amplification primers, and 2.5 U Taq polymerase, and amplified by 1 cycle of 94 °C for 3 min; 38 cycles of 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min; and the final elongation reaction at 72 °C for 5 min. The PCR products were analyzed on 1.0% agarose gels in Tris-borate-acetate buffer and visualized by ethidium bromide staining.
Total liver RNA was extracted from 100 mg liver samples from rats tolerized only, rats tolerized and transplanted with human hepatocytes, rats tolerized and transplanted with human hepatocytes followed by HBV inoculation. Samples obtained at 1, 6 and 14 weeks following HBV inoculation were homogenized in a guanidinium isothiocyanate solution[5]. RNA concentrations were determined by spectrophotometric measurement, and samples either used immediately or stored at -80 °C. In some experiments, total RNA was passed through an oligo-dT cellulose column and poly A(+) RNA isolated[6]. 10 μg total RNA or 1 μg poly A(+) RNA was reverse transcribed using reverse transcriptase (Gibco/BRL Life Technologies) with random primers. One third of the reverse transcriptase reaction sample was used for PCR generation of a 355 bp fragment using the same primers as mentioned above. One third of total RNA was mixed with 100 pmol of amplification primers, and 2.5 U Taq polymerase, and amplified for 1 cycle at 95 °C for 5 min; 38 cycles at 95 °C for 5 min, 55 °C for 1 min, 72 °C for 1 min; and the final elongation reaction at 72 °C for 5 min. The PCR products were analyzed on 1.0% agarose gels in Tris-borate-acetate buffer and visualized by ethidium bromide staining.
Immunohistochemical staining was performed as described previously[1]. In brief, frozen rat liver tissue slides were immersed in acetone for 2 min at 25 °C and washed with PBS (pH7.4). Endogenous peroxide activity was blocked by incubation in a solution containing hydrogen peroxide (Sigma) and methanol for 20 min at 25 °C. Slides were washed with PBS (pH7.4), and non-specific activity was blocked by incubation in PBS (pH7.4) and 1% BSA (Sigma) for 10 min at 25 °C. Primary antibody, rabbit anti-HBV core (Sigma), was diluted 1: 1000 in PBS (pH7.4) and 1% BSA and slides were incubated with primary antibody for 3 hrs at 25 °C. Washes were performed using PBS (pH7.4) and 1% BSA with 1% Tween-20 (Sigma). Secondary antibody, rhodamine conjugated anti-rabbit IgG (Jackson ImmunoResearch Lab., West Grove, Pa), was diluted 1:200 in PBS (pH7.4) and 1% BSA. Slides were incubated for 1 hour at 25 °C and washed with PBS and 1% BSA with 1% Tween-20, and finally washed in PBS (pH7.4). Slides were sealed and immunofluorescence visualized using a Zeiss Scanning laser confocal microscope (Model LSM-410).
Low molecular weight DNA was isolated using fractionation as described by Yeh, et al[7] and originally by Hirt[8]. 5 μg of extracted DNA were subjected to the following conditions: (a) no treatment or (b) digestion with 0.1 unit of Mung Bean nuclease (New England Biolabs, Beverly, MA) in 50 mM sodium acetate (pH5.0), 30 mM NaCl, 1 mM ZnCl2 at 25 °C for 5 min and inactivation at 70 °C for 15 min. Samples were then mixed with 100 pmol primers in 1 × PCR buffer (Invitrogen, Carlsbad, CA), 500 mM MgCl2, 100 mM dNTPs, and 2.5 U Taq DNA polymerase (Invitrogen). The cycler was programmed for 5 min at 95 °C, 2 min at 55 °C, 38 cycles of 1 min at 95 °C, 1 min at 55 °C, 1 min at 72 °C with a final cycle of 5 min at 72 °C. Samples were analyzed on a 1% agarose gel stained with ethidium bromide. The primers used were: a 21-mer sense strand (5’AGGCTGTAGGCACAAATTGGT3’) and a 20-mer antisense strand (5’GTATGGTGAGGTGAGCAATG3’) spanning 1780 to 2060 of the adwR2 HBV genome which generates a predicted 280 bp fragment after PCR.
To detect possible liver damage following inoculation of HBV, 10 μl aliquots of rat serum were assayed for ALT spectrophotometrically using kits (Sigma) as instructed by the manufacturer. All assays were performed in triplicate and results expressed as means ± SD in units of IU/L.
Liver specimens were fixed in formalin, and stained with hematoxylin and eosin to evaluate liver architecture, and cellular reaction, and examined in a blinded fashion.
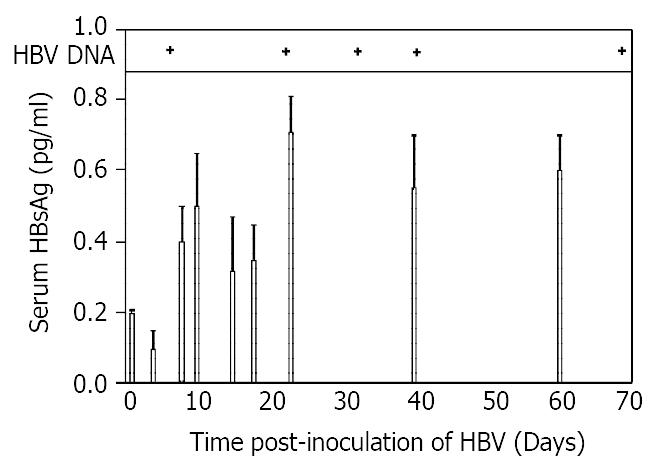
Figure 1 showed the results of assays of serum for HBsAg and HBV DNA as a function of time after inoculation of HBV into rats tolerized and transplanted with human hepatocytes cells. Surface antigen levels were detectable at a level of 0.2 pg/ml on day 1, declined slightly by day 4, but increased to a maximum of 0.7 pg/ml by day 21. There was no significant change in levels thereafter as the levels plateaued through day 60. Serum HBV DNA was detected on day 7, and remained positive on up to day 70 of post-inoculation. These data confirmed the results from our previous study[1], and were presented here to show the dynamics of the parameters in a single figure.
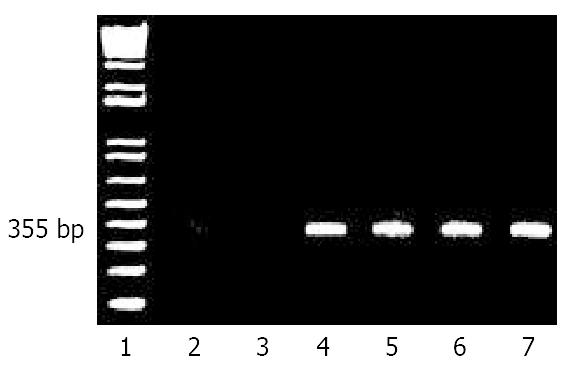
To be certain that the HBsAg detected in serum was actually produced in the liver, and not simply remnants of injected virus, the presence of viral messenger (poly A(+) RNA) was sought in liver by RT-PCR as a function of time after inoculation. Figure 2 showed that, as expected, neither normal untreated rat liver, lane 2, nor Huh7, a transformed human liver cell line, lane 3, produced any signal corresponding to the expected 355 bp fragment from amplification of HBV RNA in the core-polymerase reading frames. As a positive control, RNA from HepG2 2.2.15 cells which constitutively produced infectious HBV particles, lane 4, did produce a band of the predicted size. Livers from rats tolerized and transplanted with human hepatocytes and subsequently inoculated with HBV, also produced a band at 355 bp when assayed after one week, lane 5. This signal remained detectable at week 6, lane 6, and through week 14, lane 7.
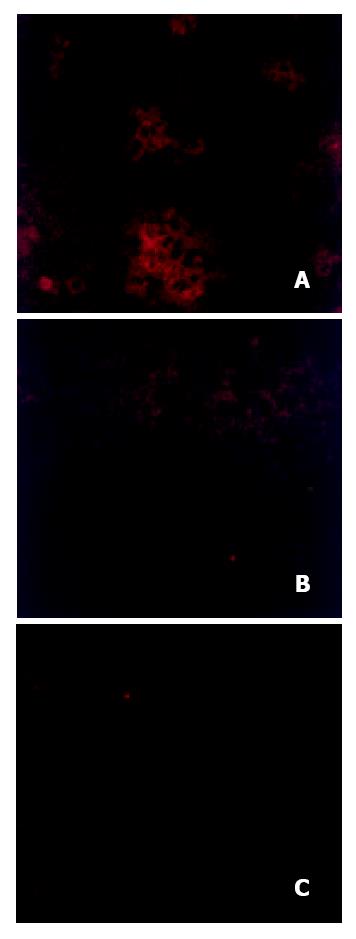
To obtain evidence of HBV replication, the presence of HBcAg was sought by immunofluorescence. Figure 3 showed a representative immunofluorescence pattern from the liver of a rat tolerized and transplanted with human hepatoctyes, and subsequently inoculated with HBV. Clumps of cells can be seen with cytoplasmic staining for HbcAg, Figure 3A. In contrast, livers from rats without transplantion, but inoculated with the same amount of HBV as the rat in Figure 3A had no staining, Figure 3B, indicating that the observed staining in Figure 3A could not have been due to uptake or artifact from the input injected virus. Similarly, control untreated rats in Figure 3C had no HBcAg staining confirming that the observed results in Figure 3A were not due to non-specific cross reactivity to a rat antigen.
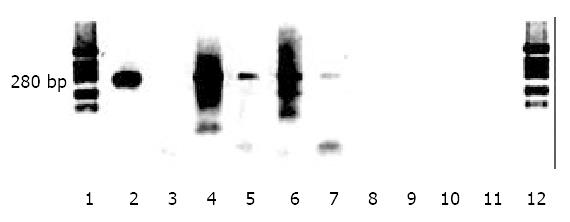
Further evidence supporting HBV replication was obtained from assays for HBV covalently closed circular DNA (cccDNA). In these experiments, Mung Bean nuclease, a DNA nuclease with a cleavage preference for single-stranded DNA was used to distinguish partially double stranded viral DNA from completely double stranded cccDNA. At low concentrations of enzyme, the partially double-stranded region DNA of the virus should be digested, and only cccDNA should survive exposure to the nuclease. In Figure 4, lane 2 showed that media from HepG2 2.2.15 cells known to secrete intact virus into the medium, produced after amplification, a band corresponding to an expected size of 280 nt. However, lane 3 showsed that when first digested with Mung nuclease, and then amplified, no signal could be produced even with 5 times the amount of DNA sample from media compared to lane 2. DNA extracted from cell layers of HepG2 2.2.15 cells produced a smear when amplified as shown in lane 4. However, prior exposure to Mung nuclease yielded a surviving band at 280 nt indicating that Mung nuclease resistant DNA was present in the low molecular weight extracts of the cell layers. Lane 6 showed that DNA extracted from livers of rats tolerized, transplanted and inoculated with HBV also generated a smear in the absence of nuclease. However, lane 7 showed that even after prior exposure to Mung nuclease, a 280 nt band spanning the partially double stranded region in the virus was still evident, supporting the presence of cccDNA in that rat liver. In contrast, DNA from rats without transplantion, but inoculated with the same amount of HBV DNA failed to generate any signal with, lane 9, or without nuclease, lane 8, indicating the signal detected in lane 7 was not due to an artifact of input viral DNA. Similarly, liver from untreated rats failed to generate the 280 nt band either with or without nuclease digestion, lanes 11, and 10 respectively, indicating that the observed band in lane 7 was not due to nonspecific amplification of host rat sequences.
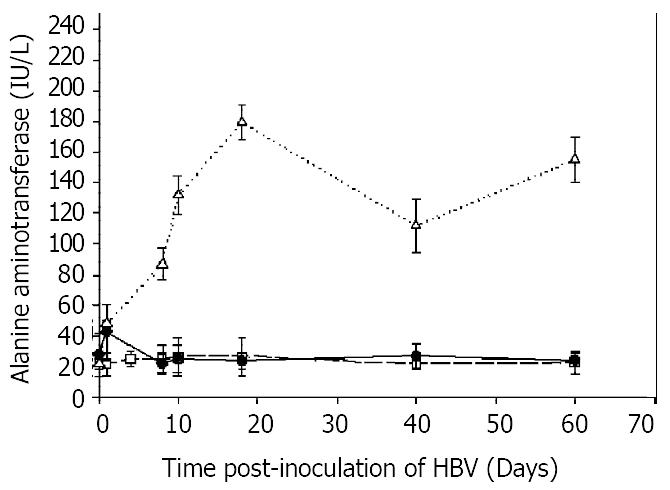
To determine whether HBV inoculation was associated with liver damage, serum alanine aminotransferase (ALT) activities were measured as a function of time after inoculation. Figure 5 showed that in animals that were transplanted with human hepatocytes, and inoculated with HBV, ALT levels rose from normal levels of 20 IU/L on the first day to a peak of 180 IU/L on day 18, and remained significantly above normal throughout the study with a value of 160 IU/L on day 60. In contrast, rats which were not transplanted, but were inoculated with HBV, had a slight increase in ALT on the first day, but returned to normal levels throughout the course. Rats that were transplanted, but not inoculated with HBV, had no increases in ALT levels at any time.
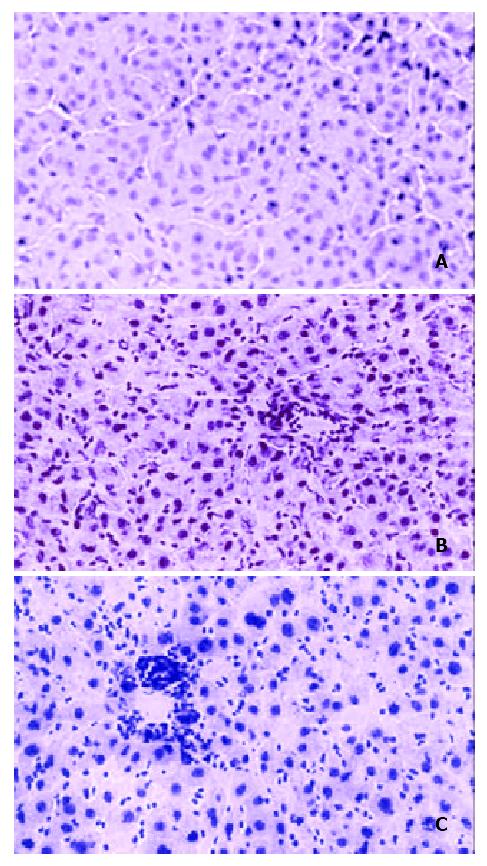
To determine whether the biochemical abnormalities were associated with any pathological evidence of liver damage, livers were examined histologically as a function of time after HBV inoculation. Figure 6A showed no significant abnormalities at one week after inoculation. However, by the sixth week, Figure 6B, there was moderate inflammation characterized by lymphocytic and neutrophilic infiltration of the lobules, Kupffer cell hyperplasia, and mild portal inflammation. Figure 6C showed that at 14 weeks of post-inoculation, the inflammation was maintained, with characteristics similar to that seen in Figure 6B: moderate lobular hepatitis with lymphocytic and neutrophilic infiltration in the lobules and portal inflammation. There were no significant differences in fibrosis among any of the samples. Control livers from rats transplanted, but not inoculated with HBV, or rats not transplanted, but were inoculated with HBV failed to show any inflammation (data not shown). These data taken together with the ALT measurements indicated that inoculation of HBV into rats transplanted with human hepatocytes was associated not only with viral replication, but also liver damage mediated at least in part by a host immune response.
The developing immune system in the fetus allows a unique opportunity for the induction of tolerance to specific cells without generalized suppression of the immune system. In utero injection of donor cells directly into the peritoneal cavities of fetuses during gestation has been shown previously to result in a donor-specific tolerance[9-11].
Chimeric liver models have been described previously, but in immunocompromised hosts. For example, normal adult woodchuck hepatocytes were transplanted into uPA/RAG2 knock out mice via intrasplenic injection. These cells eventually reconstituted 90% of those mouse livers[12]. Similarly, immortalized primary human hepatocytes have been transplanted into RAG-2 knock out mice[13]. Primary human hepatocytes transplanted under the kidney capsule of SCID/NOD mice were shown to be susceptible to HBV infection[14,15]. Recently, primary human hepatocytes were transplanted into SCID-Alb-uPA mice and shown to be susceptible to hepatitis C viral infection[16]. The results demonstrate that when transplanted into immunocompromised hosts, human hepatocytes can not only survive, but also maintain susceptibility to human hepatitis viruses. Our current data on infection and HBV replication confirmed this observation, and further indicated that general immune deficiency or suppression was not required, and intrafetal tolerization was sufficient to prevent rejection. As the mouse models described above were immunodeficient, no inflammatory response was observed as expected, in contrast to our results.
The sustained elevated levels of serum HBsAg, liver HBV RNA and serum ALT levels through 60 days of observation were not characteristics of an acute infection. Given that the viral inoculations were performed shortly (two week) after birth, the situation may be analogous to perinatal HBV infections in man. In these cases, the frequency of progression to chronic infection is extremely high, unlike the situation in adults[17]. Some data suggest that the viral load of the inoculum is predictive of the outcome of the infection in neonates[18]. Therefore, the development of chronicity in our model may be dependent on, and possibly controlled by the amount of virus in the inoculations.
While susceptibility of transplanted human hepatocytes to HBV was not totally unexpected, the development of liver damage was. It is generally accepted that the majority of the liver damage by human hepatitis B viral infection in man is due to the host immune response. However, in the case of our model, because of the mismatch between host rat immunocytes and human hepatocyte MHC, no significant recognition or interaction between HBV antigens and immune cells was anticipated. Thus, the finding of evidence of hepatitis was surprising. However, non-specific immune-mediated toxicity has been described in other systems. The mechanism of the response observed in the present study in unknown, and is presently under investigation.
Nevertheless, the model system in its present form may be useful in the evaluation of new antiviral agents for efficacy, including those with immunostimulatory properties that can only be assessed in the presence of a functional immune system. Eventually, models such as this might also permit evaluation of hepatocyte toxicity of agents as well.
We thank Martha Schwartz for her secretarial assistance.
Edited by Xu XQ
| 1. | Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 940] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 2. | Hawk CT, Leary SL. Formulary for laboratory animals. Ames, Iowa:. Iowa State University Press. 1987;. |
| 3. | Gross-Bellard M, Oudet P, Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973;36:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1252] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 4. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39100] [Article Influence: 1028.9] [Reference Citation Analysis (0)] |
| 5. | Aviv H, Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci USA. 1972;69:1408-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3693] [Cited by in RCA: 4968] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 6. | Wu CH, Ouyang EC, Walton CM, Wu GY. Human hepatocytes transplanted into genetically immunocompetent rats are susceptible to infection by hepatitis B virus in situ. J Viral Hepat. 2001;8:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Yeh CT, Wong SW, Fung YK, Ou JH. Cell cycle regulation of nuclear localization of hepatitis B virus core protein. Proc Natl Acad Sci USA. 1993;90:6459-6463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3984] [Cited by in RCA: 4349] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 9. | Yuh DD, Gandy KL, Hoyt G, Reitz BA, Robbins RC. Tolerance to cardiac allografts induced in utero with fetal liver cells. Circulation. 1996;94:II304-II307. [PubMed] |
| 10. | Yuh DD, Gandy KL, Hoyt G, Reitz BA, Robbins RC. A rodent model of in utero chimeric tolerance induction. J Heart Lung Transplant. 1997;16:222-230. [PubMed] |
| 11. | Kline GM, Shen Z, Mohiuddin M, Ruggiero V, Rostami S, DiSesa VJ. Development of tolerance to experimental cardiac allografts in utero. Ann Thorac Surg. 1994;57:72-74; discussion 75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Petersen J, Dandri M, Gupta S, Rogler CE. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1998;95:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Brown JJ, Parashar B, Moshage H, Tanaka KE, Engelhardt D, Rabbani E, Roy-Chowdhury N, Roy-Chowdhury J. A long-term hepatitis B viremia model generated by transplanting nontumorigenic immortalized human hepatocytes in Rag-2-deficient mice. Hepatology. 2000;31:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ohashi K, Marion PL, Nakai H, Meuse L, Cullen JM, Bordier BB, Schwall R, Greenberg HB, Glenn JS, Kay MA. Sustained survival of human hepatocytes in mice: A model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat Med. 2000;6:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 686] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 16. | Chang MH, Hwang LY, Hsu HC, Lee CY, Beasley RP. Prospective study of asymptomatic HBsAg carrier children infected in the perinatal period: clinical and liver histologic studies. Hepatology. 1988;8:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Lafferty KJ, Prowse SJ, Simeonovic CJ, Warren HS. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 365] [Article Influence: 8.7] [Reference Citation Analysis (0)] |