INTRODUCTION
Hepatocellular carcinoma has been one of the most common malignant threatening human health[1-9], and much higher incidences of human hepatocellular carcinoma (HCC) are observed recently[10-18]. Gap junctional intercellular communication (GJIC) is performed by intercellular hemichannels[19], which are formed by six basic protein subunits named connexin (CX) expressed in neighboring cells[20]. Six connexins form around a central pore[21], through which adjacent cells can exchange low weight molecules of information and energy directly[22]. At least 16 members of connexin super gene family have been identified, which are divided into two groups according to amino acid sequence. GJIC mediated via gap junctions plays important roles in tissue homeostasis, embryonic development as well as carcinogenensis[23-25]. The mechanisms of carcinogenesis may be related with the block of GJIC of homologous and heterologous communication between tumor cells and surrounding normal cells[26].
Connexin32 (CX32) and connexin43 (CX43) are the major kinds of gap junction proteins expressing in hepatocytes[18]. Our works showed that expressions of CX32, CX43 proteins decreased in hepatocellular carcinoma tissues and cell lines whereas increased in normal tissues and cell lines[19-21], but CX32 mRNA and CX43 mRNA kept a high level in both hepatocellular carcinoma and normal liver cells lines described previously[22,23]. It suggested that the mechanism involved in abnormal processes in post-translation or signal transduction is worthy of discussing[24].
MATERIALS AND METHODS
Cell culture
Hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal hepatocyte cell line QZG, were kindly provided by professor Chen in 863 Project Group in Fourth Military Medical University. The cells were cultured in RPMI 1640 medium (Gibco BRL, USA), supplemented with 10 mL/L fetal bovine serum (Gibco BRL, USA), incubated in a humidified atmosphere which contains 950 mL/L air and 5 mL/L CO2 at 37 °C.
Scrape loading dye transfer (SLDT)
Functions of gap junctional intercellular communication (GJIC) in adjacent cells were assessed by scrape loading dye transfer (SLDT) with fluorescent dye Lucifer Yellow (LY, Sigma, USA). The cultures were rinsed three times with serum free RPMI 1640 medium, several scrapes were made on the monolayer using a surgical scalpel, cells were exposed to 100 g/L LY in 0.33 mol/L LiCl, incubated for 3 min, and rinsed by serum free RPMI 1640 medium for several times to remove any background fluorescence. The GJIC function in adjacent cells revealed by LY dye transfer could be detected under fluorescent microscope (Nikon UFX-II, Japan).
Concentration of intracellular calcium ([Ca2+]i) measured by laser scanning confocal microscopy (LSCM)
Digested by trypsin and collected, HHCC, SMMC-7721 and QZG cells were loaded by 100 nmol/L special fluorescent dye indicator of calcium Fluo-3 AM (Sigma, USA) in serum free RPMI-1640 medium, 37 °C for 90 min, rinsed by Ca2+ free D-Hank’s for three times. MRC-1024 laser scanning confocal microscope imaging system (Bio-Rad, USA) was used to obtain the images determining the location and intensity of intracellular Ca2+ in various cell lines.
MRC-1024 laser scanning confocal microscope imaging system consists of a laser scanning system intermediate optics and a detector controlled by lasership software (Bio-Rad, USA). Images were obtained by Zesis 100 microscope and images of interest were saved to a 512 × 512 pixel, Zoom was 4.0. Besides analysis of location of Ca2+, quantities of intracellular concentration [Ca2+]i were counted with formation below: [Ca2+]i = kd × (F - Fmin)/(Fmax - F), kd = 400 nmol/L.
Immunoblot analysis of phosphorylation on tyrosine
Western blot was performed to analyses the phosphorylation on tyrosine. Cells were rinsed with cold 0.01 mol/L PBS containing 5 mmol/L EDTA, 10 mmol/L NaF and 1 mmol/L PMSF on ice for 20 min, harvested by scraping in the same solution, and centrifuged at 13000 r/min at 4 °C for 30 min. Pellets were rinsed dissolved in 20 g/L SDS. Concentrations of proteins were measured by the Bio-Rad DC protein assay (Bio-Rad, USA).
Twenty ug protein of various cells in equal amounts of 2 × SDS denatured at 100 °C for 4 min. 100 g/L SDS-PAGE vertical electrophoretically Min-PROTEAN II (Bio-Rad, USA) was used at 80V for 30 min and 120V for 60 min. Mini Trans-Blot transferred proteins form gel to NC membranes (Hybond, USA) by transferring electrophoretically (Bio-Rad, USA) at 100V 350 mA 4 °C for 2 h.
Normal serum blocked NC membranes at room temperature for 2 h. CX32 and CX43 protein bands were detected using a mouse monoclonal anti-CX32 antibody (1:2000, Zymed, USA), a mouse monoclonal anti-CX43 antibody(1:2000, Zymed, USA). Phosphorylations on tyrosine were detected by special anti-phosphorylation tyrosine mAb 4G10 (1:2000, CA, USA) at room temperature for 2 h. NC membranes were washed by TBST for 10 min for several times, incubated with a biotin-streptavidin alkaline phosphatase conjugated secondary IgG (1:32, Johnson, USA) at room temperature for 2 h and washed by TBST for 10 min for several times again. The enhanced chemilunimescence (ECL) detection kit (Amersham, UK) was employed to detect immunoblot signal.
RESULTS
Gap junctional intercellular communication of various cells
After stained with special fluorescent dye Lucifer Yellow (LY), function of gap junctional intercellular communication were detected by scrape loading and dye transfer (SLDT). Molecular weight of LY is below 1000 u that can pass via gap junction smoothly. Normal liver cell line QZG showed stronger intensity and amount yellow fluorescent among neighboring cells, whereas hepatocellular carcinoma cells HHCC and SMMC-7721 showed much slight fluorescent staining limited among adjacent cells.
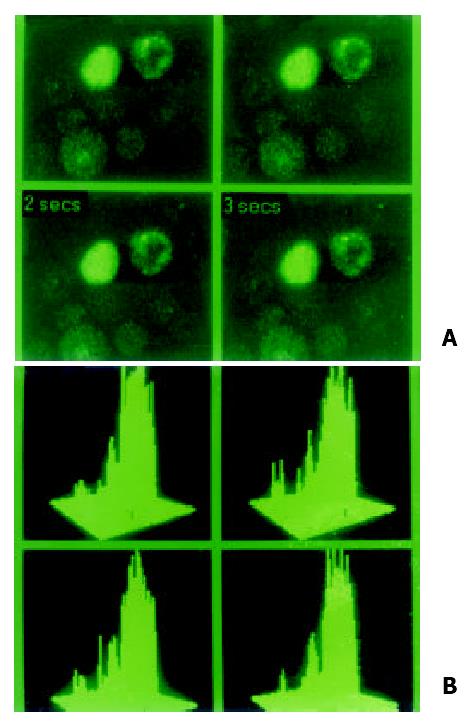
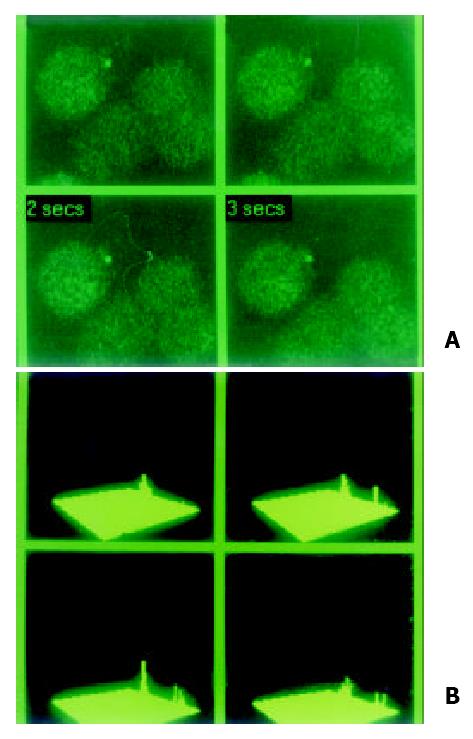
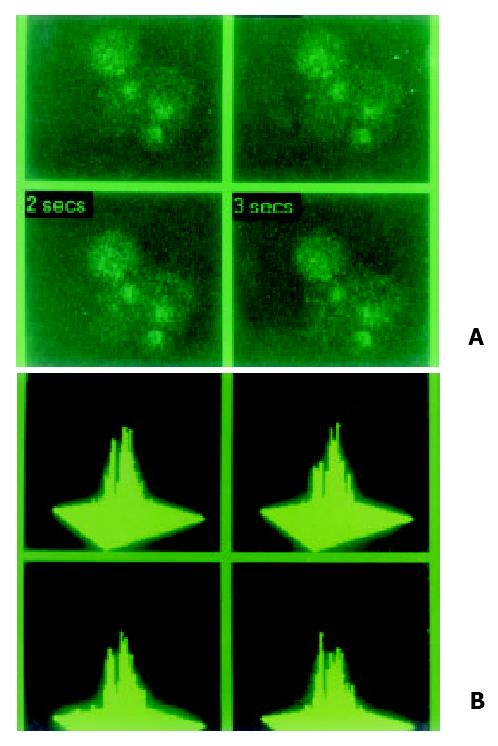
Analysis of concentrations and location of intracellular Ca2+ ([Ca2+]i) LSCM was used to determine intracellular Ca2+ in cultured cells after being loaded with special indicator of Ca2+ Fluo-3 AM. The results showed that intensity of Ca2+ was much higher with a very bright and higher peak signal of Ca2+ in QZG cells. Intracellular concentration of Ca2+ in QZG cells was 108.37 nmol/L. In HHCC and SMMC-7721 cells peak signals of Ca2+ were lower. [Ca2+]i in HHCC cell decreased to 35.13 nmol/L, SMMC-7721 cells also had lower peak Ca2+ and its [Ca2+]i decreased to 47.08 nmol/L as shown in Figure 1, Figure 2, Figure 3.
Figure 1 Special indicator Fluo-3 AM was loading at 37 °C for 90 min in hepatocarcinoma cell lines in order to examine localization and concentration of intracellular calcium in MRC-1024 laser scanning confocal microscope imaging system.
(A). In normal hepatocyte cell line QZG, stronger signal of calcium with lower peak released. (B). Concentration of intracellular calcium in HHCC was 108.37 nmol/L.
Figure 2 Special indicator Fluo-3 AM was loading at 37 °C for 90 min in hepatocarcinoma cell lines in order to examine localization and concentration of intracellular calcium in MRC-1024 laser scanning confocal microscope imaging system.
(A). In hepatocarcinoma cell line HHCC, slight signal of calcium with lower peak was observed. (B). Concentration of intracellular calcium in HHCC was 35.13 nmol/L.
Figure 3 Special indicator Fluo-3 AM was loading at 37 °C for 90 min in hepatocarcinoma cell lines in order to examine localizatio and concentration of intracellular calcium in MRC-1024 laser scanning confocal microscope imaging system.
(A). Loading by Fluo-3 AM, dark signal of calcium and lower peak was also absent in hepatocarcinoma cell line SMMC-7721. (B). Concentration of intracellular calcium was 47.08 nmol/L.
Phosphorylation on tyrosine of CX32, CX43 proteins
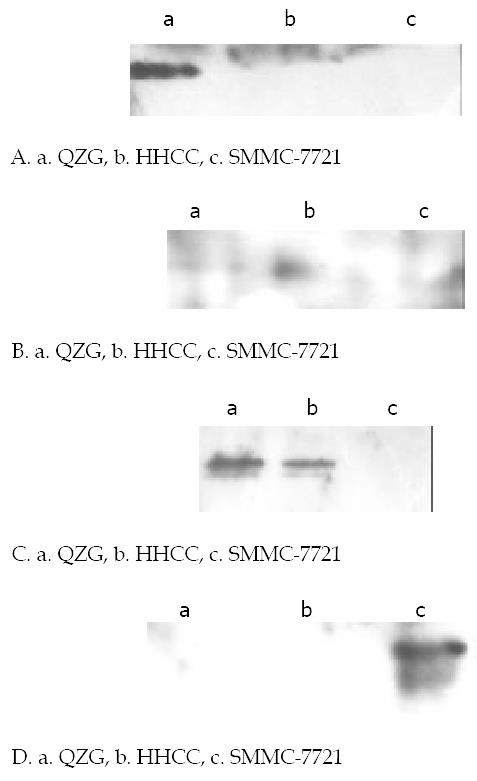
The expressions of CX32, CX43 proteins were assessed using western blot analysis with anti-CX32 mAb, anti-CX43 mAb. We also verified the phosphorylation on tyrosine using western blot analysis with anti-phosphorylated tyrosine mAb 4G10.
Western blot showed that major bands at 32 ku, 44 ku in a blot is unphosphorylated tyrosine in QZG cells. It recognized a band at 32 ku phosphorylated tyrosine CX32 protein and phosphorylated tyrosine CX43 protein of 43 ku in SMMC-7721 cells. No evidence of phosphorylated tyrosine at 32 ku of CX32 and 44 ku of CX43 proteins were detected in HHCC cells.
These results conform that the mouse monoclonal antibody selectively binds phosphorylated tyrosine CX43 proteins was corresponding decrease of concentrations of calcium and block of gap junctional intercellular communication in same hepatocellular carcinoma cell lines as shown in Figure 4A-D.
Figure 4 Western blot analysis of phosphorylation on tyrosine of CX32, CX43 proteins in various cells lines.
Cells were harvested and lysed, equal amount of cell lystates were resolved of SDS-PAGE, transferred to NC membranes, and then probed with anti-phophorylation tyrosine mAb 4G10. (A). Expressions of CX32 proteins in cell lines with special anti-CX32 mAb. CX32 showed high immunoblot signal only in normal hepatocyte cell line QZG with very slight signal in hepatocarcinoma cell lines HHCC, SMMC-7721. (B). Expressions of CX43 proteins in cell lines with special anti-CX43 mAb. CX43 apperared in both QZG and SMMC-7721 cells but no in HHCC. (C). Phosphorylation on tyrosine of CX43 with special anti-phosphrylation tyrosine 4G10, unphosphorylation appeared in QZG cells even they showed high level expression of CX32, CX43. (D). Phosphorylated tyrosine of CX43 protein was detected in SMMC-7721 cells.
DISCUSSION
Gap junctions are channels between neighboring cells, which allow small molecules, and ions (mass molecules are below 1000 u) to pass through directly. Normal hepatocytes express high quantities of connexin32, connexin43 as the major gap junction forming proteins[20,27]. As described previously, immunohistochemical analysis showed gap junctional proteins CX32, CX43 frequently decreased in hepatocellular carcinoma tissues and cell lines[28-31]. But by in situ hybridization (ISH), CX32 mRNA, CX43 mRNA in hepatocellular carcinoma tissues and cell lines showed same strong positive rates and density without significant difference[32,33]. The aberrant location of CX32 and CX43 proteins might be responsible for hepatocarcinogenesis[34], the mechanism might be defect of CX genes in post-translational processing[35].
In various neoplasm, including hepatocellular carcinoma cells often express less connexin, but the mechanisms are unknown. It suggested that decrease of expression and lacking of function of CX32, CX43 might keep close relationship with liver hepatocarcinogenesis. Disruption of GJIC activity could contribute to the multi-step, multi-mechanism process of carcinogenesis[36]. Most tumors demonstrate a reduction between either homologous and heterologous gap junctional intercellular communication (GJIC). GJIC was assessed by transfer of the fluorescent dye Lucifer Yellow (LY) after scrape loading dye transfer (SLDT) in hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG. Molecular mass of LY is below 1000 u, which can pass via gap junction smoothly[37]. Our results showed that QZG had high ability of GJIC function than that in HHCC and SMMC-7721 cell lines, which were almost blocked.
Presumably down-regulation of GJIC activity would lead to the removal of growth inhibitory signaling. It was hypothesized that an initiated cell would be growth suppressed by being coupled by gap junctions to surrounding normal cells. Disturbance of GJIC facilitate cells to escape from intercellular signals involved regulations on proliferation, differentiation and apoptosis. Many factors, tumor-promoting chemicals[38] and oncogenes influence signal transduction in the initiated and surrounding normal cells, which would cause the down-regulation of GJIC. Many kinds of tumor promoting agents have been shown to decrease expressions of gap junction proteins or block GJIC associated with hepatocarcinogenesis[39]. H-ras, v-myc also cooperate malignancy[40]. Connexin genes might belong to the group of tumor suppressor genes.
Our results suggested that multiple mechanisms likely contributed to block of GJIC, including decrease of expressions of gap junction proteins and abnormal pathway of signal transduction including deceased level of intracellular Ca2+, phosphorylation on tyrosine and other potential mechanisms. In different tissues molecules smaller than approximately 1000 u may pass through gap junctions which implies that the most common second messengers cAMP, inositol-1,4,5-trisphosphate (IP3) and intracellular free calcium ions Ca2+ can be transferred. The mechanisms of intracellular calcium signaling are currently being investigated. Mechanisms during hepatocarcinogenesis are related to multiple pathophysiological processes including decreased in intracellular Ca2+. Calcium signals might be an important mechanism for controlling and synchronizing physiology. With intact gap junctional coupling, calcium in connected hepatocytes can communicate directly through gap junctions.
Whereas, gap junctional permeability and conductance affected by the calcium concentrations spikes in hepatocytes, a reduction of junctional conductance by calcium concentrations has been observed in hepatoma cells[42,43]. In our study, after special indicator of calcium Fluo-3AM loading, LSCM was used to measure concentrations of intracellular calcium [Ca2+]i in hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG. Concentrations of intracellular calcium [Ca2+]i was much higher in QZG (108.37 nmol/L) cell line than that in HHCC (35.13 nmol/L) and SMMC-7721(47.08 nmol/L) cell lines. QZG cells showed much higher calcium peaks than HHCC, SMMC-7721 cells which was associated with disruption of GJIC in these cell lines. Decreased level of intracellular Ca2+ contributed to conduction abnormalities of GJIC which immediate loss of calcium fluxes and influence the calcium dynamics in hepatocellular carcinoma cell lines.
Gap junctions are modulated by a multi-step molecular components and signaling pathway. Our observations also suggested that disruption of GJIC in hepatoma cells was associated with multiple distinct steps such as phosphorylation on tyrosine of CX32, CX43 proteins[43]. CX contains phosphorylations sites for several protein kinases including protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) which are involved in CX phophorylation leading to the closure of GJIC[44,45]. Disruption of GJIC requires CX43 phosphorylation and interaction with other cellular factors. Phosphorylation of CX proteins has been postulated to be a critical regulatory element for gap junctional communication[46]. In our study, western blot suggested that only QZG cells had unphosphorylated tyrosine in CX32 protein at 32ku and CX43 protein at 43ku; SMMC-7721 cells had phosphorylated tyrosine CX43 proteins. Change in the amount and distribution of phosphorylated and nonphosphorylated isoforms of CX32 and CX43 were measured by immunoblot using isoform-secific antibodies anti-CX32 mAb, anti-CX43 mAb. Dramatic decreases in gap junctinal communication and loss of phosphorylation on tyrosine[47]. It indicated post-translation phosphorylation on tyrosine of CX proteins, which associated with a dramatic disruption of GJIC[48]. Functional recovery was associated with increased levels of unphosphorylated CX43.
As above all, disruption of GJIC in hepatocellular carcinoma cells could also be achieved by multi-mechanisms. We could make conclusions that the establishment and permanent of functional gap junctions were regulated by transcriptions as well as post-translational controls including reduce of concentrations of intracellular calcium and phosphorylation on tyrosine of connexin proteins even the biological co nseq uences o f sp ecific chang es in CX p ro tein s phosphorylation are not understood in detail. The decrease of CX proteins or the disorder of signal transduction of CX genes might keep close relationships with hepatocellular carcinoma.