INTRODUCTION
Although MHC-antigens have been extensively studied, the problem of rejection in liver transplantation was not resolved completely. Liver transplantation are not strict for HLA matching and both grafts and recipients can survive for a long time after immunotherapy[1]. On the other hand, given HLA multiple loci matching in some cases, the likelihood of grafts dysfunction and rejection increased, which suggested that matching for HLA type may exert a dualistic effect on liver transplantation[1,2]. No satisfactory explanations have been made about it up to date; and most studies were limited to HLA antigens themselves with the results still remaining controversial.
The specific antigens expressed only in liver cell membrane or liver cytoplasma and encoded by loci not linked to the MHC gene were called liver specific antigen (LSA). It was reported that this antigen could be detected in sera of almost all liver allotransplantation recipients but its effects remained unknown yet[3]. Thus in this experiment, we aimed to find whether LSA was an transplantation antigen and its possible mechanism of inducing the tolerance of liver allotransplantation using intrathymic LSA inoculation pathway. And the roles of LSA in thymus tolerance were discussed in this research too.
MATERIALS AND METHODS
Materials
Inbred male Wistar and male SD rats weighing 200 to 250 g were purchased from Shanghai Experimental Animal Center; there were also materials used in this study as following: [γ-32P]ATP (Furui bioengineering Corp, Beijing); EMSA assay kit (Promega); NF-κB double stranded oligonucleotide, 5’-AGTTGAGGGGACTTTCCCAGGC-3’, 3’-TCAACTCCCCTGAAAGGGTCCG-5’ (Santa Cruz), NF-κB double stranded mutant oligonucleotide, 5’-AGTTGAGG-CGACTTTCCCAGGC-3’, 3’-TCAACTCCGCTGAAAGGGTCCG-5’ (Santa Cruz); TRIzol Reagent (Gibco, BRL); MuLV (MBI, Fermentas); Ultracentrifugator (Beckman); CHRIST lyophilizer (B.Braun Biotech).
Methods
Isolation of LSA Preparation of liver specific protein S100 was carried out by the method previously described[4]. The protein contents was measured according to the method of Bradford. Then the protein was lyophilized by CHRIST lyophilizer and stored at -80 °C.
Surgical procedure, experimental groups and sample harvesting Rats were anesthetized with ether inhalation. Orthotopic rat liver transplantation was performed by Kamada’s two-cuff technique[5]. The animals surviving less then 3 d after the transplantation were attributed to technical errors and were excluded from this study. Wistar rats serving as recipients were randomly divided into four groups, Group I: syngeneic control (Wistar-to-Wistar); Group II: acute rejection (SD-to-Wistar); Group III: acute rejection treated with intramuscular injection of CsA 3.0 mg/kg/d (SD-to-Wistar + CsA); Group IV: Intrathymic inoculation of 10 mg SD rat LSA one week before transplantation (LSA + SD-to-Wistar). All groups were subdivided into day 1, 3, 5, 7, 12 subgroup (n = 3 each) after the transplantation respectively for sample harvesting and another subgroups (n = 6) for observation of common situation and survival time. For observation of common situation and survival time in Group III, after the therapy of CsA for 12 d, animals received no additional immunosuppression. Graft specimens were harvested to determine morphological changes and cytokine gene expression. Recipient splenocytes were isolated and the NF-κB activity were determined.
Histopathological examination Grafted liver samples were fixed in 10% buffered formalin and embed in paraffin. Five micrometers think sections were affixed to slides, deparaffinized, and stained with hematoxylin and eosin to assess morphologic changes and severity of acute rejection by the Kemnitz’s standard[6].
Cytokine reverse transcription-polymerase chain reaction Primer sequences and reaction conditions: Primers sequences used were as follow: IL-2 sense primer, 5’-GACGCTTGTCCTCCTTGTCA-3’, IL-2 antisense primer, 5’-ACCACAGTTGCTGGCTCATC-3’ (size 372 bp); IFN-γ sense primer, 5’-ACTGCCAAGGCACACTCATT-3’, IFN-γ antisense primer, 5’-AGGTGCGATTCGATGACACT-3’ (size 235 bp); β-actin sense primer, 5’-TCGTACCACTGGCATTGTGA-3’, β-actin antisense primer, 5’-TCCTGCTTGCTGATCCACAT-3’ (size 645 bp). Amplifications were performed under the following conditions: 95 °C for 2 minutes, 94 °C for 45 seconds, 56 °C for 45 seconds, 72 °C for 45 seconds, totally 32 cycles. The final extension step was performed by one cycle at 72 °C for 10 minutes. Reaction buffer: 10 × Buffer 2.5 µl, 10 mM dNTP 1 µl, MgCl 2 µl, cDNA 2 µl and 1 µl of each primer, ddH2O was added to get the final volume of 25 µl. RT-PCR: Total RNA was prepared from grafted liver with TRIzol Reagent according to the manufacturer’s recommendations. For cDNA synthesis, 4 ug total RNA was reverse transcribed with MuLV reverse transcriptase according to the manufacturer’s recommendations. Two microliters from the resulting cDNA solution were then amplified using specific oligonucleotides under the reaction conditions using β-actin as a “housekeeping gene” in a volume of 25 ul PCR buffer. Reaction products were run by electrophoresis on a 1.5% agarose gel for 30-40 min at 100 V in 0.5 × Tris/borate/EDTA buffer, and visualized with ethidium bromide under UV light. Relative expression of cytokines were defined as optical density ratio (cytokine/β-actin) analyzed by Kodak digital science scanning system.
NF-κB activity of splenocytes and EMSA competitive inhibition NF-κB activity was detected by gel electrophoretic mobility shift assay (EMSA): Splenocytes of recipients were isolated and nuclear extracts were prepared according to the method described by Kravchenko[7]. The contents of protein were determined by the method of Bradford. NF-κB oligonucleotide was end-labeled with [γ-32P]ATP according to the manufacturer’s recommendations. For EMSA, 10 ug of each nuclear extract was mixed with 5 × binding buffer at room temperature for 10 min. Then 1 ul [γ-32P] ATP-labeled double strand NF-κB oligonucleotide probe was added in and incubated at room temperature for 20 min. The DNA/protein complex was electrophoresed on 4% nondenaturing polyacrylamide gels in 0.5 × Tris/borate/EDTA buffer to separate probe binding to NF-κB and free probe. Radioactive bands were detected by autoradiography at -70 °C followed by radiography to detect the level of retardation. The results were expressed as relative optical density (ROD)[8]. To study whether the method of EMSA was specific, standard nuclear extract of Hela cells was used to bind with NF-κB protein. The specificity of binding was confirmed by 100 fold excess unlabeled NF-κB oligonucleotide as a specific competitor, 100 fold excess unlabeled non-related oligonucleotide as a nonspecific competitor and 100 fold excess unlabeled mutant NF-κB oligonucleotide as a mutant competitor. All unlabeled oligonucleotides were preincubated with Hela nuclear extract at room temperature for 10 min then labeled NF-κB oligonucleotide was added. The left steps were the same as mentioned above.
Statistics analysis
All data were expressed as mean ± SD and analyzed by one-way repeated measures analysis of variance (ANOVA) using SPSS software (version 11.0 for windows). Pearson correlation analysis was used between parameters. P < 0.05 was considered as statistically significant.
RESULTS
Postoperative common situation and survival time
The common situation of rats in the Wistar-to-Wistar group was very good after the transplantation. Recipients drank normally after 3rd day of posttransplantation. Normal coat recovered at day 7 with increase of body weight and all recipients survived for a long time. Recipients of rats in the SD-to-Wistar group ate badly with tarnished coat and progressive loss of body weight; all died within 9 to 13 d after the transplantation; median survival time was 10.7 ± 0.51 d. Although 5 of 6 recipients of rats in the SD-to-Wistar + CsA group survived for a long time (one dead from abdominal inflammation and liver abscess day on 14 after the transplantation), they also drank badly with tarnished coat and ceased increase of body weight during CsA medication, which recovered after the cease of drug treatment. As for the LSA + SD-to-Wistar group, 5 of 6 recipients survived for a long time (one dead of bile duct obstruction on day 15 after the transplantation) and their common situation were remarkably better than that of rats in the SD-to-Wistar group and SD-to-Wistar + CsA group.
Histopathologic examination
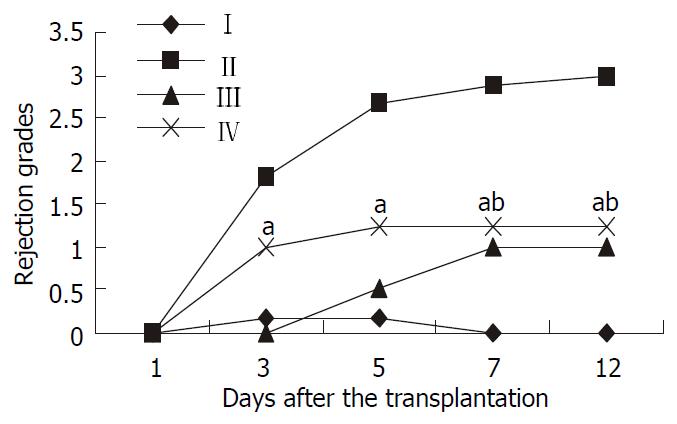
No signs of rejection were found at any time point in the Wistar-to-Wistar group with minimal portal inflammatory infiltrates. In the SD-to-Wistar group, a few portal lymphocyte infiltrations combined with minimal vein endothelialitis on day 1 was found but there was no evidence of rejection. Significant portal lymphocyte infiltration with degeneration of hepatic parenchyma in some cases can be found on day 3 and day 5 with average rejection grade of 1.83 and 2.67, respectively. Severe inflammatory cells infiltration, severe vein subendothelialitis with bridging hepatocellular necrosis could be found on both day 7 and day 12 with rejection grade of 2.87 and 3, respectively. Owing to the immunosuppressive effect of CsA, rejection was greatly inhibited in the SD-to-Wistar + CsA group. No evidence of rejection was found at the first three time points. But mixed inflammatory infiltration and endothelialitis could be found on day 7 and day 12, in which grade 1 rejection was diagnosed. As for the LSA + SD-to-Wistar group, rejection grades was 0 and 1 on day 1 and 3, respectively. Mixed but mainly lymphocytic infiltration was found in both portal tract and parenchyma without focal necrosis of the hepatic parenchyma on day 5, 7 and 12. Thus their rejection grades were all defined as 1.25 which were significantly lower than that in the SD-to-Wistar group (P = 0.026). Furthermore, no significant discrepancies of rejection grades on day 7 and day 12 were found between the the SD-to-Wistar + CsA group and LSA + SD-to-Wistar group (P = 0.067) (Figure 1).
Figure 1 Rejection grades of grafted liver in different groups.
Data were expressed as mean ± SD. I, Wistar-Wistar group; II, SD-Wistar group; III, SD-Wistar + CsA group; IV, LSA + SD-Wistar group; Rejection grades of group IV were significantly lower than that in group II at all but day 1 time point. No sig-nificant discrepancies were found between group IV and group III on day 7 and day 12 time points. aP < 0.05 vs group II, bP > 0.05 vs group III.
Expression of cytokine gene
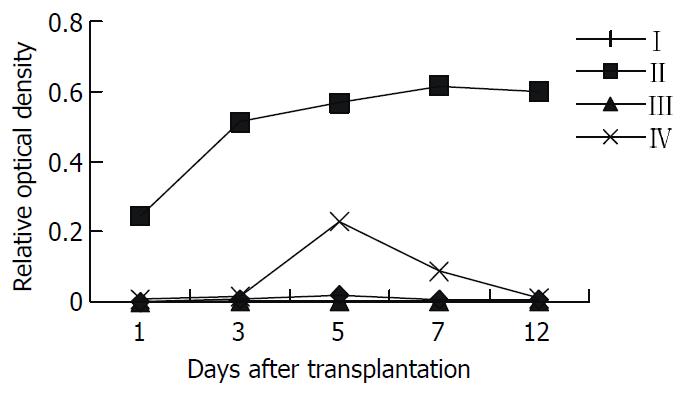
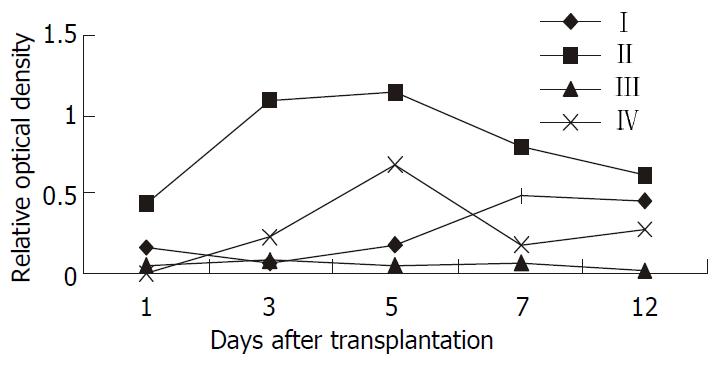
The kinetic changes of intragraft IL-2 mRNA expression in different groups were shown in Figure 2: no expression was detected at any time point in the Wistar-to-Wistar and the SD-to-Wistar + CsA group, whereas high level expression was detected at all time points in the SD-to-Wistar group and reached to the peak on day 7 and 12 after the transplantation (P < 0.001, vs the Wistar -to-Wistar group). Low level expression was only detected on day 5 in the LSA + SD-to-Wistar group which was significantly lower than that in the SD-to-Wistar group (P < 0.001). Intragraft IFN-γ mRNA expression in different groups was shown in Figure 3. Very weak IFN-γ mRNA expression was detected at any time point in the Wistar-to-Wistar group after the liver transplantation, whereas there was very strong expression in the SD-to-Wistar group at all time points (P = 0.006, vs the Wistar-to-Wistar group). The expression of IFN-γ mRNA was significantly inhibited in the SD-to-Wistar + CsA group which was significantly lower than that in the SD-to-Wistar group (P < 0.001). As for group IV, its kinetic change was similar to that in the SD-to-Wistar group but was significantly lower than that in the SD-to-Wistar group at any time points (P = 0.046). Expression of IL-2 and IFN-γ mRNA was correlated to the histopathological damage.
Figure 2 The kinetic change of intragraft IL-2mRNA expres-sion in different groups.
I, Wistar-Wistar group; II, SD-Wistar group; III, SD-Wistar + CsA group; IV, LSA + SD-Wistar group; IL-2mRNA expression of group IV were significantly lower than that in group II at all time point (P < 0.05).
Figure 3 The kinetic change of Intragraft IFN-γ mRNA ex-pression in different groups.
I, Wistar-Wistar group; II, SD-Wistar group; III, SD-Wistar + CsA group; IV, LSA + SD-Wistar group; IL-2mRNA expression of group IV were significantly lower than that in group II at all time point (P < 0.05).
EMSA specific competitive inhibition and NF-kB activity of splenocytes
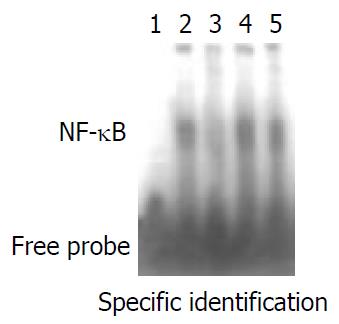
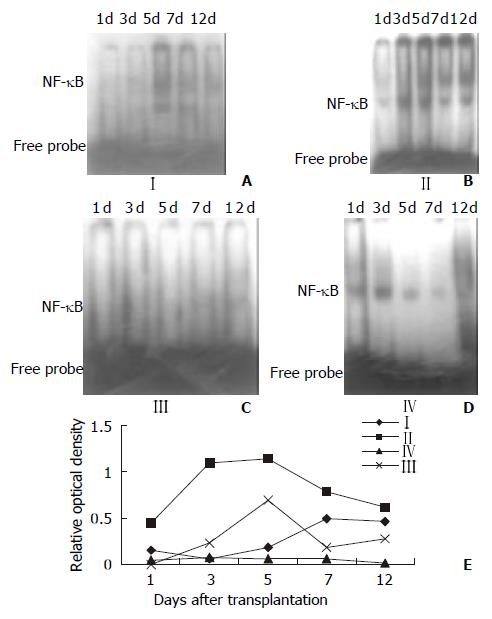
The results of EMSA competitive inhibition showed that no NF-κB activity was found after the specific competition, whereas NF-κB activity was the same as the positive control after the nonspecific competition and mutant competition (Figure 4). This results showed that this method was specific. NF-κB activity of splenocytes in Figure 5 showed the autoradiographic results of splenocyte NF-κB DNA binding activity in different groups (A, B, C, D). Low NF-κB activity was only detected on day 5 and day 7 in the Wistar-to-Wistar syngeneic group after the transplantation. In contrast, NF-κB activity increased from day 3 after the transplantation in the SD-to-Wistar group and was significantly higher than that in the syngeneic group at all time points (P = 0.001). Only very weak DNA binding activity was found on day 12 in the SD-to-Wistar + CsA group and NF-κB activity was also inhibited in the LSA + SD-to-Wistar group which was significantly lower than that in the SD-to-Wistar group (P = 0.003). NF-κB activity was significantly inhibited in the SD-to-Wistar + CsA and LSA + SD-to-Wistar group (Figure 5E).
Figure 4 EMSA specific competitive inhibition.
Lane 1, nega-tive control; Lane 2, positive control; Lane 3, specific competition; Lane 4, non-specific competition; Lane 5, mutant competition.
Figure 5 NF-κB activity of splenocytes at different time points after the transplantation.
A, Wistar-Wistar group; B, SD-Wistar group; C, SD-Wistar + CsA group; D, LSA + SD-Wistar group; E, the kinetic change of NF-κB activity. NF-κB activity of group IV were significantly lower than that in group II at all time point (P < 0. 05).
Correlation analysis
A good correlation was found between the activity of NF-κB and intragraft IL-2 as well as IFN-γ mRNA expression in this study (pearson correlation analysis, r = 0.737 and r = 0.742 respectively, P < 0.01, n = 20). While intragraft IL-2 and IFN-γ mRNA expression were correlated to histopathological damage (pearson correlation analysis, r = 0.856 and r = 0.680 respectively, P < 0.01, n = 20).
DISCUSSION
Allotransplantation tolerance can be induced by intrathymic inoculation of donor cellular antigen[9-12], and also by intrathymic inoculation of donor soluble MHC antigen[13-15]. However, intrathymic inoculation of donor soluble non-MHC antigen have not been reported heretofore. In this study, we inoculated donor LSA into recipient thymus before the liver transplantation so that rejection was greatly inhibited. Only mild rejection was found in grafts and was significantly lower than that in the non-LSA inoculated group and similar to that in the CsA treated group. Recipients survived for a long time due to the induction of immunotolerance.
Although the rejection grades in the LSA + SD-to-Wistar group was increased obviously from day 3 and peaked at day 5 after transplantation, there was no significant difference between time points except for day 1 (P > 0.05). This rejection kinetic change in the LSA + SD-to-Wistar group was similar to that in the Wistar-Wistar group and was significantly lower than that in the SD-to-Wistar group (P = 0.026). Furthermore, the rejection grades in the LSA + SD-to-Wistar group did not significantly differ from that in the SD-to-Wistar + CsA group on day 7 and day 12 time point after the transplantation (P = 0.067), which showed that intrathymic inoculation of LSA could greatly alleviate rejection. However, rejection in the SD-to-Wistar group was increased continuously and the rejection grade on day 3 after the transplantation was significantly different from its peak one (P < 0.05). It was an optimal acute rejection control. As for the SD-to-Wistar + CsA group, rejection was greatly suppressed due to the effect of CsA and recipients survived for a long time, which was consistent with previous reports[16]. The rejection grades in all but SD-to-Wistar + CsA group was increased rapidly within groups when compared with that on day 1 after the transplantation (Figure 1) showed that the high immunoresponses starting from day 3 to day 5 after the transplantation was the “rejection crisis” phase[17]. Rejection crisis phase in the SD-to-Wistar + CsA group was postponed to start from day 5 to day 7 after the transplantation due to the strong immunosuppressive effect of CsA. Meanwhile, that rejection grades was not increase on day 7 after the transplantation both in the SD-to-Wistar + CsA group and LSA + SD-to-Wistar group and recipients survived for a long time suggested that the pathological damage in these two groups may be “self-limited”. Given longer harvesting time point, more could probably be found.
For the expression of IL-2mRNA, the most related cytokine to rejection, detected using RT-PCR in grafts, low level expression was only detected on day 5 in the LSA + SD-to-Wistar group and it was significantly lower than that in the SD-to-Wistar group (P < 0.001) (Figure 2). Although the expression of IFN-γ mRNA, another important cytokine to rejection, was slightly higher than that in the Wistar-to-Wistar group (P = 0.427), it was also significantly inhibited in the LSA + SD-to-Wistar group (P < 0.046, vs SD-to-Wistar group) (Figure 3). IL-2 and IFN-γ are the representative cytokines secreted by Th1 cells which play important roles in transplant rejection[18-20]. Both intragraft IFN-γ mRNA and protein were increased when grafts were rejected[21,22]. IFN-γ not only increases expression of MHC class I and class II antigens of intragraft, but stimulates cells without expression of MHC class II antigens at quiescent state to express MHC class II antigens[23]. Thus inhibiting the synthesis and secretion of IL-2 and IFN-γ is one of the mainly target for preventing transplantation rejection all the while.
As an important nuclear transcription factor, NF-κB is a specific sequence binding protein which can bind to promoters or enhancers of multiple genes including closely related cytokines and adhesion molecules to organ transplantation rejection[24], thus extensively regulating the expression of these genes. Apart from that the change of NF-κB activity exerts crucial effects on the proliferation and activation of immune cells, NF-κB is also an important antiapoptotic nuclear factor[25,26]. So the change of NF-κB activity may play a crucial role in the immunoresponses and directly influence the immunoreaction after the transplantation. In its quiescent state, NF-κB is localized in cytoplasm in a complex with its inhibitory proteins (IκBs)[27,28], which mask its nuclear localization signal and prevent its from translocation to the nucleus. NF-κB is activated through phosphorylation and IκBs is subsequently degraded and then translocated into nucleus to bind with specific DNA sequences to regulate the expression of related genes[26]. NF-κB regulates the transcription of IL-2 mRNA through binding to its binding sites in down-stream of IL-2 gene promoter. It has been demonstrated in vitro that activated NF-κB can directly result in high level expression of IL-2 mRNA and promote the proliferation and activation of T lymphocyte[29]. IFN-γ gene belongs to lymphokine gene family and is related to transplantation rejection. Its transcription parallels that of other lymphokine genes such as IL-2 whose promoter activity can be enhanced by NF-κB protein[30,31]. In this regard, the ubiquitous factor NF-κB controlling a number of cytokines may have roles in the transcription of IFN-γ gene. It has also been demonstrated that NF-κB interacts with NF-AT to coregulate the gene expression of IFN-γin vitro[32] but not in vivo especially in the organ transplantation rejection. We used rat orthotopic liver transplantation model to study the relationship between the activity of NF-κB and the expression of IL-2 and IFN-γ mRNA and its mechanisms with or without CsA and LAS treatment. A good correlation was found between the activity of NF-κB and the expression of intragraft IL-2 as well as IFN-γ mRNA in this study (pearson correlation analysis, r = 0.737 and r = 0.742 respectively, P < 0.01, n = 20). While the expression of intragraft IL-2 and IFN-γ mRNA were correlated to the histopathological damage (pearson correlation analysis, r = 0.856 and r = 0.680 respectively, P < 0.01, n = 20). Thus it is believable that allotransplantation rejection is partly due to the activation of NF-κB at least. Both CsA and intrathymic inoculation of donor LSA can inhibit the activity of NF-κB and further inhibit the expression of IL-2 and IFN-γ mRNA and rejection after the transplantation.
We preliminarily discuss the specific tolerance mechanisms induced by LSA: T lymphocytes recognition process of tissue specific antigens is MHC-restricted which includes direct and indirect recognition and the latter is the main one. In this experiment, before the transplantation, protein antigen was used to inoculate recipient thymus. No APC and MHC molecules of donor existed, so the recognition of LSA should be indirect. Modern immunology believes that TCR recognizes neither MHC molecules nor antigen peptides alone. What it recognizes is surface information of MHC-antigen peptides complex. So it is believed that although LSA is organ specific, space conformation, electric charge qualities and distributions of MHC-antigen peptides complex are not the same due to its polymorphism, thus activating allolymphocytes[33]. But large numbers of activated lymphocytes may be clonally deleted due to high dose antigen immunization, which coincides with the “high-dose/activation-associated tolerance” mechanism[34]; In addition, thymus reeducation may also be one of the mechanisms of this tolerance.T lymphocytes of recipient recognize donor LSA as self by reeducation mechanism.
Therefore it was postulated that given multiple MHC loci matching, the processing ability of tissue specific antigens by recipient MHC molecules will be enhanced or be equal to that of by donor’s, thus bring about indirect recognition of T lymphocytes changing into direct recognition and increasing the occurrence of hepatopathy and rejection after the transplantation. This may be one of the reasons why mutiple MHC loci matching, especially DR matching, can increase the rates of grafted liver dysfunction and rejection[1,2]. This may be just as what Desquenne postulated that it should make it easier to induce tolerance of skin antigen as well as other tissue specific antigen when MHC incompatibilities prevail[35]. Besides, special attention must be paid to the LSA + SD-to-Wistar group. The common situation of recipients without any transient immunosuppression were much better than that in the non-LSA or CsA-treated group and no such complications as inflammations happened. It showed that the tolerance did not result from down-regulation of body’s immunofunction, in contrast recipients’ immunuofunction was normal. This is perhaps the optimal condition to induce donor specific tolerance and do not affect body’s immunofunction. Reasonable explanation is that strong immunoreaction towards to liver parenchymal cells happened when transplanted liver was rejected. Thus the tolerance induced by LSA is towards to hepatocytes and is in real sense a donor and organ specific one.
The results of this experiment not only demonstrated that thymus had unusual effects on the tolerance induction, but also that allotolerance to non-MHC antigen can be induced after contacting of thymus microenvironment to non-MHC antigen. It may suggest that if immunodominant character or immunodominant non-MHC antigens were identified, differences of other MHC and non-MHC antigen may be neglected, thus leading to long-term allografts survival across complete MHC barrier. It also suggested that MHC antigens may be not the only obstacles to successful transplantation. It is reported here for the first time that intrathymic inoculation of LSA can induce permanent and specific immunotolerance of liver allotransplantation, which show that hepatocytes are directly involved in the immunoreaction of liver transplantation. It’s an important supplement to the traditional theory of liver transplantation rejection which considers that rejection is mainly involved in liver vascular bed, bile ducts and nonparenchymal cells, thus leading to a novel way to liver transplantation immunotolerance.