INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide, especially in several areas of Asia and Africa. It is the cause of death of around 1000000 people annually. Although many new methods appeared as palliative protocols in the past 30 years, patient survival after onset of symptoms remained dismal[1-3]. Surgical treatment such as resection or orthotopic liver transplantation is potentially curative only for patients with small and localized HCC[4]. Transfer of therapeutic genes to tumor mass or to the peritumoral tissues provides a promising new approach for cancer therapy[5-8]. Both viral and non-viral vectors were used to transfer genetic material to the interior of target cells[9]. Experiments demonstrate that tissue and transcriptional targeting expression of transgenes in tumor cells not only improves the outcome of treatment, but also reduces systemic toxicity[10]. This results in a much higher therapeutic index. Alpha-fetoprotein (AFP) is over expressed in about 70% of HCC cases[11]. Several groups have used AFP promoter or/and enhancer to control foreign gene expression in different vectors[12,13]. We constructed a gene modified AFP cis-acting element using the minimal essential DNA segments of AFP gene enhancer and promoter. Specific transcription activity of this element and it’s response to the addition of ATRA were tested using reporter gene vector pEGFP-1.
MATERIALS AND METHODS
Reagents
Reagents were obtained as follows: EX Taq DNA Polymerase, DNA Ligation Kit Ver.2, restriction endonuclease (TakaRa Biotechnology (Dalian) Co. Ltd.); DNA isolation and Purification kit (ShangHai ShunHua Biotechnology Ltd.); LipofectamineTM 2000 (Invitrogen); IPTG, X-gal, DNA maker (Sino-American Biotechnology Company); Dulbecco’s Modified Eagle Media: high glucose, with L-glutamine (DMEM) and G418 (GibcoBRL); all-trans retinoic acid, ATRA (Sigma).
Plasmids
pBluescript II ks(+) vector was preserved in our laboratory. The reporter gene vector pEGFP-1 was purchased form Clontech. This vector encoded a red-shifted variant of wild-type green fluorescent protein (GFP), which had been optimized for brighter fluorescence and higher expression in mammalian cells. This vector was also a non-promoter EGFP vector which could be used to monitor transcription from different promoters and promoter/enhancer combinations inserted into the MCS located in upstream of the EGFP coding sequence.
Cell lines
HepG2 and SMMC-7721 are human hepatoma cell lines produce or do not produce AFP. Hela is human cervical cancer cell line, all Cell lines were cultured in Dulbecco’s modified Eagle’s medium, high glucose content, containing 10% heat-inactivated fetal calf serum, 100 units/mL penicillin, 100 µg/mL streptomycin, 0.292 mg/mL glutamine. The cell lines were incubated in a humidified 5% CO2 and 95% air incubator at 37 °C.
Polymerase chain reaction (PCR)
The primer sequences used for PCR were used as reported[14,15]. For PCR amplification, genomic DNA were extracted from 1 × 106 HepG2 cells and digested by EcoR I/Xho I. PCR was performed in a total volume of 50 µl consisting of 1 µM each primer, 200 µM each dNTP, 5 µL 10 × polymerase reaction buffer, and 1.25 U EX Taq DNA Polymerase with 1 µL digested DNA. The samples were heated to 94 °C for 5 min followed by amplification for 30 cycles of 30s at 94 °C, 50s at 55 °C, and 1 min at 72 °C. After the last cycle, a final extension step was done at 72 °C for 7 min. Then 10 µl of each PCR product was analyzed by 1% agarose gel (containing 0.5 µg/mL EB) electrophoresis. After which the amplified PCR products were purified from agarose gel according to DNA purification kit description. The abbreviated gene fragments of AFP enhancer (e) and promoter (p) were subcloned into pBluescript II ks(+). The positive clones were selected from the transfected DH5α and performed as described in reference[16]. The constructed plasmids (designated as ks-e, ks-p) were identified by restriction enzyme analysis. DNA sequences were verified by DNA Sequencing Core Facility in Bioasia (ShangHai) Biotechnology Company.
Construction and identification of expressing vector pEGFP-1-EP
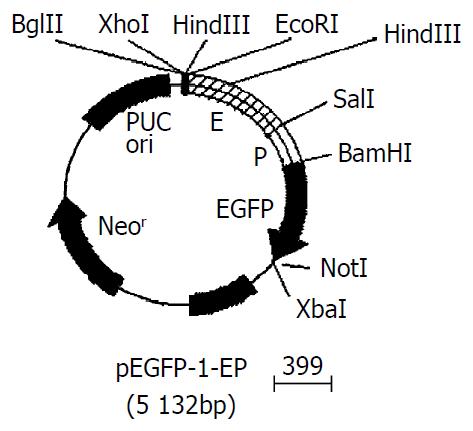
Gene fragments of AFP enhancer (e) and promoter (p) were released by EcoR I-Sal I, and Sal I-BamH I double digestions respectively, and inserted into the EcoR I- BamH I sites of pEGFP-1. The Recombinant was identified by restriction endonuclease digestion. The restriction maps of pEGFP-1-EP were shown in Figure 1.
Figure 1 Gene map of pEGFP-1-EP.
Transient EGFP expression
18 hours before transfection, 2 × 105 HepG2, SMMC-7721 and Hela cells were plated into 12-well culture plates. At the bottom of each well, a sterilized coverslip was placed in advance. When the plated cells were 90 to 95% confluence, transfection was performed with 1 µg of DNA (pEGFP-1 or pEGFP-1-EP) per dish, using LipofectamineTM 2000 reagent according to the manufacturer’s instruction. 48 h after transfection, the cells were washed twice with cold PBS (0.01 M) and fixed with 4% paraformaldehyde for 30 min at room temperature. Coverslips were then mounted directly onto a glass slide with a tiny drop of 50% glycerol in PBS (9.1 mM Na2HPO4/1.7 mM NaH2PO4/50 mM NaCl, pH7.4). Fluorescent images were captured at 490 nm using a Nikon Eclipse E1000 microscope attached to a MicroMax camera (Princeton Instruments, Trenton, NJ).
Effect of all-trans retinoic acid (ATRA) on the expression of EGFP
A similar transfection procedure was perfomed to transfect vector pEGFP-1-EP for stable EGFP expression. After 48 h of transfection, the cells were passaged at a 1:4 dilution into fresh medium. The next day, selective medium (G418400 µg/mL) was added to the cells for screening of stable lines of transfected cells. After culturing for an additional 2 weeks in G418, cells expressing EGFP fluorescence were selected using a fluorescence-activated cell sorter. The EGFP-positive cells were maintained in growth medium supplemented with 400 µg/mL G418. For RA regulation studies, stably transfected cells were plated on 12-well dishes at 1 × 105 cells/well in growth medium with G418, incubated overnight, then changed into the medium with or without all-trans retinoic acid (1 × 10-7 M, 2.5 × 10-7 M, 1 × 10-6 M ATRA) (without G418). The expression rate of EGFP fluorescence was measured by flow cytometry (ETITE ESP, COULTER Company) 48 h later. The green fluorescence was analyzed through a 530-nm/30-nm band pass filter after illumination with the 488-nm line of an argon ion laser.
Statistical analysis
All experiments were performed three times in triplicate, the data were presented as the mean ± SEM, and compared by Student’s t test.
RESULTS
PCR amplification and DNA sequencing of gene modified AFP enhancer and promoter
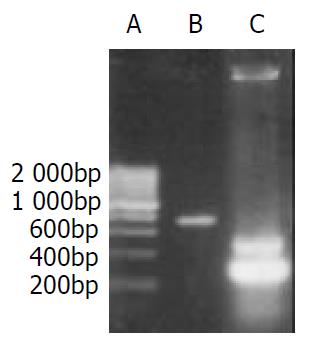
Electrophoretic result of PCR products showed that the size of amplified DNA fragments was consistent with design. Through PCR amplification of AFP promoter we obtained two gene fragments (0.3 kb, 0.5 kb). The 0.3 kb fragment was what we expected, the 0.5 kb one was nonspecific amplification (Figure 2). PCR products were subcloned into pBluescript II ks(+) vector. The DNA Sequencing results confirmed that DNA sequence of both fragments was correct.
Figure 2 PCR result of gene segments.
A. 200 bp DNA marker; B. enhancer of AFP; C. promoter of AFP.
Gene modified AFP cis-acting element was successfully inserted into the MCS of pEGFP-1
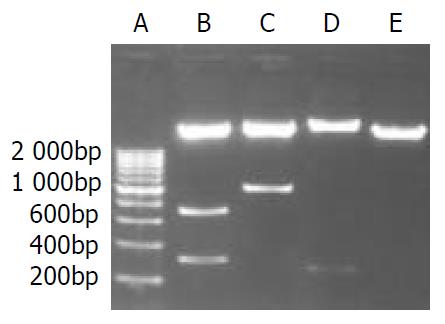
Recombined AFP cis-acting element was 1035 bp with EcoR I and BamH I restriction site on each side. The size of pEGFP-1 was 4.2 kb, which had Bgl II and Hind III sites in the 3’-side of MCS. The expressing vector pEGFP-1-EP was digested by Bgl II/Sal I/BamH I, and Xho I/BamH I, and Hind III respectively. 0.73 bp-0.31 bp, and 1.03 bp, and 0.27 bp DNA fragments appeared (Figure 3). This result meant that the size, direction of cis-acting element were correct and the construction of expressing vector was successful.
Figure 3 Enzyme digestion analysis of the recombinant ex-pression vector.
A. 200 bp DNA marker; B. pEGFP-1-EP (Bgl II, Sal I, BamH I); C. pEGFP-1-EP (Xho I, BamH I); D. pEGFP-1-EP (Hind III); E. pEGFP-1(EcoR I, BamH I).
Specific expression of EGFP in AFP positive hepatoma
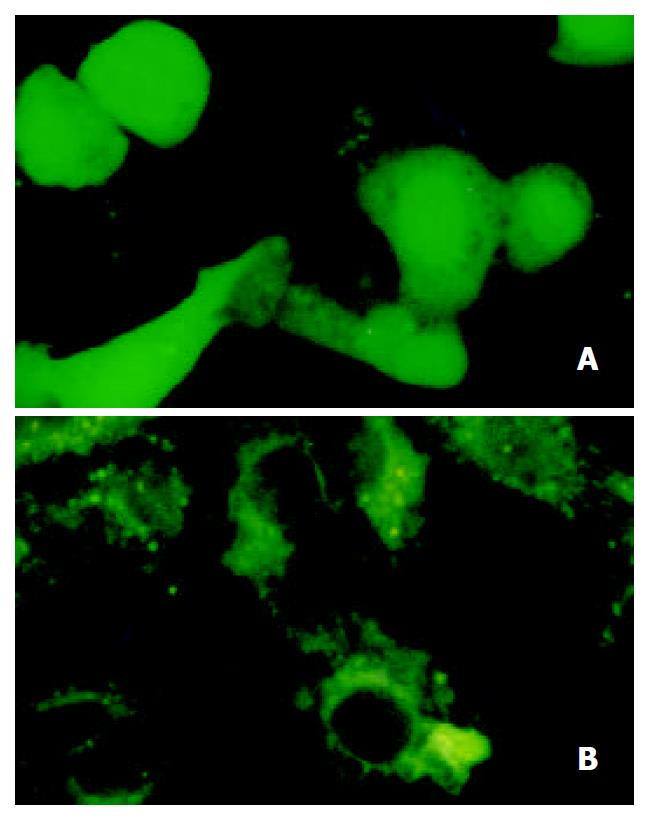
Three cell lines were transfected with plasmid pEGFP-1 and pEGFP-1-EP simultaneously. The expression of EGFP could only be detected in HepG2 cells (AFP positive) transfected with pEGFP-1-EP (Figure 4). The transient expression rate of EGFP in HepG2 was 16%. Hela cell and SMMC-7721 cell (AFP negetive) did not express EGFP. The HepG2 cell transfected with pEGFP-1 also did not express EGFP. So we could conclude that gene modified cis-acting element retained specific transcription activity in AFP positive cells.
Figure 4 Transient expression of EGFP.
A. EGFP expressing in HepG2; B. EGFP do not express in Hela cells.
The expressing of EGFP can be suppressed by the adding of ATRA
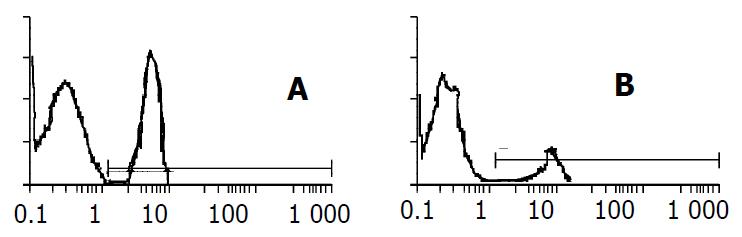
EGFP-expressing HepG2 cell line was obtained through two weeks screening by adding G418 into the culture medium. These cells were then cultured in the medium with or without retinoic acid for 48 h and measured by flow cytometry. The expression rate of EGFP was 34.9% ± 4.1% in cells without retinoic acid and 14.7% ± 3.5%, 13.5% ± 2.7%, 12.1% ± 3.9% in cells adding 1 × 10-7 M, 2.5 × 10-7 M, 1 × 10-6 M retinoic acid (Figure 5). Transcription activity of modified AFP cis-acting element was suppressed by adding either concentration of retinoic acid (P < 0.05).
Figure 5 The expression of EGFP suppressed by ATRA.
A. stable expression of EGFP in HepG2; B. expression of EGFP suppressed by 2.5 × 10-7 M ATRA.
DISCUSSION
There are numerous gene therapy strategies that have been applied to the treatment of HCC. They include gene replacement (e.g. tumor suppressor genes), antisense strategies, drug sensitization (suicide genes), genetic immunotherapy (cytokines, costimulatory molecules, polynucleotide vaccination), and interventions to interfere with the biology of the tumor growth (antiangiogenesis)[17-19]. But HCC frequently occurs in patients with liver cirrhosis, the potentially toxic effects of gene transfer may have marked deleterious consequences, so the targeted gene transfer or the specific expression of transfected genes to hepatoma cells is vital for gene therapy of HCC.
A practical system to ensure tumor-restricted expression of the transgenes is the use of tumor-specific promoters. Since elevated levels of AFP have been observed in about 70% of hepatocellular carcinomas[20], using AFP transcriptional sequence to achieve hepatocellular carcinoma specific gene expression is an ideal way. Arbuthnot et al[21,22]. demonstrated that retroviral and adenoviral vectors containing the lacZ reporter gene under the control of AFP regulatory sequences resulted in the specific expression of the lacZ gene only in HCC cells with AFP expression. Similar results were reported by Kanai et al[23]. who demonstrated that expression of the herpes simplex virus thymidine kinase (HSV-tk) gene by adenovirus from AFP promoter/enhancer induced the cells to be sensitive to ganciclovir (GCV) in the AFP-producing cells. But The AFP gene has a large and complex transcription control region which is located within a region from -7.6 kb to the transcription starting site. It contains the promoter, the enhancer, and the silencer that contain specific binding sequences for the transcription factors and provide precisely regulated AFP gene transcription[24,25]. Most investigators have used human AFP 5’-flanking region that including the all the trans- and cis-acting elements from AFP 5’-flanking sequences[24,26-29] as a hepatoma specific regulation element. Although this sequences can achieve sufficient cytotoxicity in AFP-producing hepatoma cells, but also induce a killing effect on hepatic stem cells that also express AFP and appear in the injured liver, thus resulting in damage of the hepatic reserve and a poor prognosis for patients with HCC. On the other hand, the package capacity of most gene therapy vectors is very small, which cannot accommodate the whole transcriptional sequence of AFP in the construct. The activity of promoter alone is too weak to be used in targeting gene therapy. In this study, we subcloned the minimal essential enhancer region (-4.0 to -3.3) and 0.3 kb minimal promoter of human AFP gene, and constructed abbreviated HCC specific cis-acting element. The expressing of the report gene (EGFP) was detected in AFP-producing hepatoma cells (HepG2), and none in non-AFP-producing hepatoma (SMMC-7721) and nonhepatoma cells (Hela). So this minimal cis-acting element well conserved the specific transcription characteristic of AFP 5’-flanking sequences. This element is very small (1.0 kb) and fits to the package capacity of most conventional gene therapy vectors.
The expression of AFP is under the control of retinoic acid which both activates and represses AFP expression in different cell lines[30,31]. Within the AFP gene regulatory region three elements determining sensitivity for retinoic acid have been revealed. One of them is localized in promoter (-139/-127 bp) and overlaps with other transcription factor binding sites[31]. The influence of retinoic acid on AFP gene expression can be carried out both by means of HNF induction and through the hormone-receptor complex binding to the corresponding sites in AFP regulatory elements[32]. Activation of the AFP gene by RA had been reported in McA-RH8994 and F9 cell lines[30,31], while AFP was down-regulated by RA in the human hepatoma cell line Hep3B. How can RA regulate the expression of AFP in HepG2 human hepatoma cell line is rarelys reported. In this study we established an EGFP-expressing HepG2 cell line, the expressing of EGFP is under the control of modified cis-acting element of AFP. When treated with ATRA, we found that the transcription activity of AFP cis-acting element could be significantly suppressed by 1 × 10-7 M ATRA. So when we use this transcription element as a regulator of anti-tumor gene the cytotoxicity effects on target cell can be controlled by RA within the expected range. This is very important when a gene therapy vector is applied clinically.
There are restriction sites in the upstream or downstream of EGFP in the expressing vector pEGFP-1-EP, through which we can replace EGFP with any tumor-killing gene. So this vector can be used as a gene therapy vector targeting for hepatocellular carcinoma. The transcription activity of hepatocellular carcinoma specific promoter can be controlled by the adding of ATRA.