Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.674
Revised: November 2, 2002
Accepted: November 9, 2002
Published online: April 15, 2003
AIM: To evaluate the expression of cyclooxygenase (COX-2) and the relationship with tumor angiogenesis and advancement in gastric adenocarcinoma.
METHODS: Immunohistochemical stain was used for detecting the expression of COX-2 in 45 resected specimens of gastric adenocarcinoma; the monoclonal antibody against CD34 was used for displaying vascular endothelial cells, and microvascular density (MVD) was detected by counting of CD34-positive vascular endothelial cells. Paracancerous tissues were examined as control.
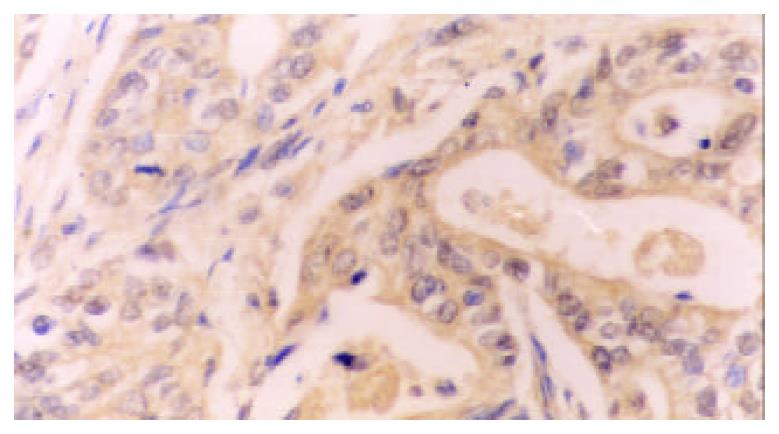
RESULTS: Immunohistological staining with COX-2-specific polyclonal antibody showed cytoplasmic staining in the cancer cells, some atypical hyperplasia and intestinal metaplasia, as well as angiogenic vasculature present within the tumors and prexisting vasculature adjacent to cancer lesions. The rate of expression of COX-2 and MVD index in gastric cancers were significantly increased, compared with those in the paracancerous tissues (77.78 vs 33.33%, 58.13 ± 19.99 vs 24.02 ± 10.28, P < 0.01, P < 0.05, respectively). In 36 gastric carcinoma specimens with lymph node metastasis, the rate of COX-2 expression and MVD were higher than those in the specimens without metostasis (86.11 vs 44.44%, 58.60 ± 18.24 vs 43.54 ± 15.05, P < 0.05, P < 0.05, respectively). The rate of COX-2 expression and MVD in the specimens with invasive serosa were significantly higher than those in the specimens without invasion to serosa (87.88 vs 50.0%, 57.01 ± 18.79 vs 42.35 ± 14.65, P < 0.05, P < 0.05). Moreover, MVD in COX-2-positive specimens was higher than that in COX-2-negative specimens (61.29 ± 14.31 vs 45.38 ± 12.42, P < 0.05). COX-2 expression was positively correlated with MVD (r = 0.63, P < 0.05).
CONCLUSION: COX-2 expression might correlate with the occurance and advancement of gastric carcinoma and is involved in tumor angiogenesis in gastric carcinoma. It is likely that COX-2 by inducing angiogenesis can be one of mechanisms which promotes invasion and metastasis of gastric carcinoma. It may become a new therapeutic target for anti-angiogenesis.
- Citation: Li HX, Chang XM, Song ZJ, He SX. Correlation between expression of cyclooxygenase-2 and angiogenesis in human gastric adenocarcinoma. World J Gastroenterol 2003; 9(4): 674-677
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.674
COX is a key enzyme in the conversion of arachidonic acid to prostaglandin, and two isoforms of COX, namely COX-1 and COX-2, have been identified[1,2]. COX-1 is constitutively expressed in many tissues and is considered to be involved in various physiological functions, whereas COX-2 is induced by pathological stimuli, such as inflammation, various growth factors and cytokines produced by tumor cells[1-3].
Epidemiologic studies showed that nonsteroidal anti-inflammatory drugs (NSAIDs), known to inhibit COX, could reduce the incidence rate and mortality from digestive tract carcinomas[4-10]. In rodent models of FAP, a genetic disease leading to colonic carcinoma, blockade of COX-2, suppresses intestinal polyp formation[11]. Increased COX-2 expression has been reported in colorectal, pancreatic, hepatocellular and other cancers[12-20]. Taken together, these data provide strong evidence for the importance of COX-2 in oncogenesis.
It has been reported that tumor angiogenesis play an important role in tumor growth, invasion and metastasis[21-25]. We investigated the expression of COX-2, MVD in human gastric cancer. The aim of this study was to determine the relationship between COX-2 and tumor angiogenesis,and the development, progression of gastric cancer. The further understanding of oncogenesis might provide a new approach to tumor therapy.
45 patients with gastric adenocarcinomas confirmed pathologically underwent gastrectomy in our hospital from January 2000 to October 2001. From these subjects, gastric tumor and paracancerous tissues (more than 5 cm away from the lesion) were obtained from resected specimen. Among them, 35 were male, and 10 female, with a mean age of 57.51 ± 10.73 (33 to 78). Patients who had received radiotherapy or chemotherapy before gastrectomy were excluded. Histologically, they were classified by the WHO criteria, 5 were highly differentiated adenocarcinoma, 10 moderately-differentiated, 27 poorly-differentiated, 3 undifferentiated. As regards to the size of cancer, 20 were < 5 cm, 25 ≥ 5 cm. 33 tumors invaded to the serosa and 12 tumors not.36 cases had local lymph node metastasis.
Antibody against COX-2 was purchased from Santa Cruz Biotechnology. Inc; antiboby against CD34 and ready to use SP immunohistochemical reagent box were purchased from Fijian Maixin CO, Ltd. Formalin-fixed, paraffin-embedded surgical specimens from 45 cases of gastric carcinoma were available and sliced sequentially with a thickness of 4 µm. The slices carrying the detected antigen were dyed with SP immunohistochemical staining method, and those in the control group were dyed according to the above method, with the first antibody substituted by PBS.
The data were presented as -x±s; numerical variable by χ2 test; enumeration data by t test; COX-2 relationship with MVD by spearman rank correlation test (depending on the quantitative index of COX-2 and MVD).
The cytoplasm of the gastric cancer cells stained with brown granules were identified to be COX-2 positive. Only nucleuses stained blue were identified to be COX-2 negative. COX-2 expression was scored semi-quantitatively according to the density and the percentage of positivity into score 0, 1, 2, 3. A minimum of 10 high power view were used to assess COX-2 expression level. If the sum of two scores was 1-3, the slice would be considered as low-expression of COX-2. If 4-6, it would be considered as high-expression of COX-2. Vascular endothelial cells were considered CD34-positive if their cytoplasm stained brown or brownish yellow. The microvessels were counted according to the number of single endothelial cell or endothelial cell clustor showing brownish yellow granules in the cytoplasm. The slices were observed first microscopically under the low power (× 40),then selected the most dense area of microvessels was selected to be observed under high power (× 200, the surface area of every visual field was 0.785 mm2), and the number of microvessels in 3 visual field were counted and took the average as MVD of this specimen[26].
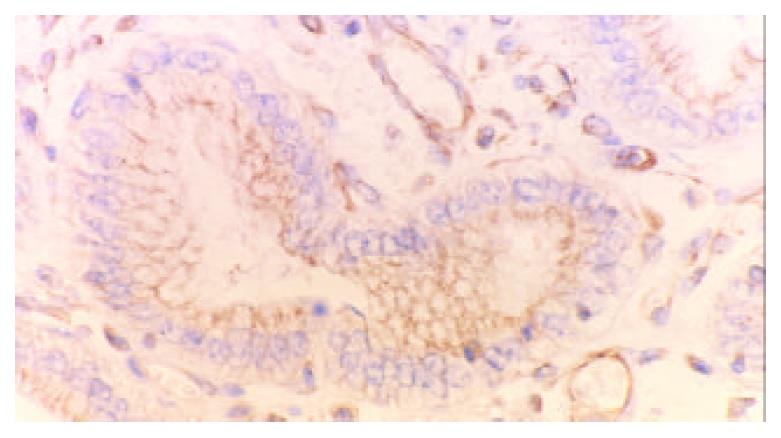
77.78% (35/45) cases of gastric carcinomas showed COX-2 positive expression while high-expression was detected in 22 cases, low-expression in 13.33.33% (15/45) cases of paracancerous tissues showed COX-2 positive expression while high-expression was only detected in 3 cases. The rate and density of COX-2 expression in cancerous tissues were significantly higher that in paracancerous tissues (χ2 = 18, χ2 = 6.09, P < 0.005, P < 0.05, respectively). The positive COX-2 staining was mainly diffusely located as brownish yellow stained granules in the cytoplasm. Immunohistological analysis revealed cytoplasmic staining in the neoplastic cells (Figure 1), atypical hyperplasia and intestinal metaplasia. In addition, COX-2 was also detected in the angiogenic vasculature present within the tumors and preexisting vasculature adjacent to cancer lesions (Figure 2). In contrast, normal epithelium or stroma occasionally showed weak staining pattern or didn’t.
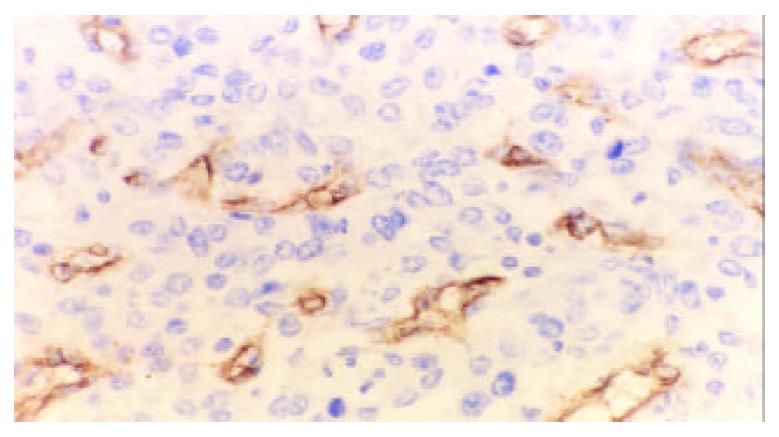
The mean MVD in gastric carcinoma was significantly higher than that in para-cancerous tissues (58.13 ± 19.99, 24.02 ± 10.28, t = 10.18, P < 0.001). The positive expression of CD34 was mainly presented as brownish yellow or brownish granules in the cytoplasm of vascular endothelial cell (Figure 3). New blood vessles in the cancerous lesions had no regular contour and were not evenly distributed.
The result showed that MVD (61.29 ± 14.31) in the COX-2-positive gastric cancerous tissues was higher than that (45.38 ± 12.43) in the COX-2-negative one (t = 5.64, P < 0.001). The expression of COX-2 was positively correlated with MVD (r = 0.63, P < 0.05).
In Table 1, the associations between COX-2, MVD expression and the pathological features were shown. Both COX-2 and MVD were not correlated with tumor size, tumor histological type. However, there was correlation between COX-2, MVD and depth of invasion and lymph-node metastasis of gastric carcinoma respectively.
| Pathological characteristics | Positive | MVD | Total |
| COX-2 (%) | -x ± s | ||
| Tumor size | |||
| < 5 cm | 15 (75.0) | 60.64 ± 18.55 | 20 |
| ≥ 5 cm | 20 (80.0) | 55.68 ± 17.98 | 25 |
| Depth of invasion | |||
| Invading serosa | 29 (87.88)a | 57.01 ± 18.79c | 33 |
| Noinvasion to serosa | 6 (50.0) | 42.35 ± 14.65 | 12 |
| Degree of differentiation | |||
| Well differentiated | 12 (80.0) | 52.45 ± 17.67 | 30 |
| Poorly differentiated | 23 (76.67) | 57.32 ± 18.20 | 15 |
| Lymph-node metastasis | |||
| Positive | 31 (86.11)b | 58.60 ± 18.24d | 36 |
| negative | 4 (44.44) | 43.54 ± 15.05 | 9 |
Human gastric mucosa normally expresses no detectable levels of COX-2 protein[27,28]. In the current study, we found that the rate of COX-2 expression in gastric cancer was significantly increased, compared with that in the paracancerous tissues, the expression of COX-2 showed cytoplasmic staining, not only in cancerous cells but also in precancerous lesion such as atypical hyperplasia and intestinal metaplasia. A similar pattern of COX-2 expression has previously been found in human gastric cancer[29-34]. The above data demonstrated that COX-2 was up-regulated in human gastric cancer, suggesting COX-2 may play an important role in occurrence of gastric cancer, being a relatively early event in the carcinogenesis of stomach.
Here, we also analyzed the relationship between COX-2 expression and clinical pathological features in gastric carcinoma. It was shown that the rate of COX-2 expression was correlated closely with the depth of tumor invasion, indicating COX-2 may contribute to invasive growth of gastric carcinoma. The rate of COX-2 expression of gastric carcinoma with lymph-node metastasis was higher than that without suggesting the increase of its expression in gastric cancer tissue can promote lymph-node metastasis. It seemed more likely that COX-2 probably heightened viability and increased infiltrative potential of gastric cancer. The mechanism was not clear. Tsujii concluded that overexpression of the COX-2 gene as a result of transfection promoted invasiveness in wild type human colon carcinoma cell lines through the induction of metalloproteinase-2 and membrane-type metalloproteinase[35]. Rat intestinal epithelial cells that overexpressed COX-2 protein were found to be resistant to butyrate-induced apoptosis and had elevated bcl-2 protein expression and decrease expression of both E-cadherin and the transforming growth factor-β receptor[36]. Each of these changes has been linked to enhanced tumorigenic potential and increased tumor invasiveness. Therefore, the above data further indicated that COX-2 might play an important role in gastric tumorigenesis and tumor progression.
Recently the relation of COX-2 and tumor angiogenesis is emphasized. One of the mechanisms by which PGE2 supports tumor growth is by inducing the angiogenesis necessary to supply oxygen and nutrients to tumors > 2 mm in diameter[37,38]. Masferrer[39] reported that SC-236, a COX-2-selective inhibitor, was effective in reducing angiogenesis driven by bFGF in the matriel rat model, whereas SC-560, a COX-1-selective inhibitor was ineffective. He also observed COX-2 expression in newly formed blood vessels within tumors grown in animals, whereas under normal physiological condibions the quiescent vasculature expressed only the COX-1 enzyme, indicating COX-2-derived prostaglandins contributed to tumor angiogenesis[40]. In our study, COX-2 expression was also detected in the angiogenic vasculature present within the tumors and preexisting vasculature adjacent to cancer lesions, suggesting COX-2 may induce newly formed blood vessels to sustain tumor cell viability and growth. COX-2 was also expressed within atypical hyperplasia, intestinal metaplasia and neovasculature in the paracancerous tissue, indicating COX-2 may promote precancerous lesion to cancer by new blood vessel formation.
MVD is a reliable index of tumor angiogenesis[41]. We found that the MVD in COX-2 positive tumors was significantly higher than that in COX-2 negative tumors, MVD in gastric carcinoma was higher than that in paracancerous tissues, suggesting its distribution was similar to the pattern of COX-2 in gastric carcinoma. A close correlation was present between MVD and COX-2 (P < 0.01), indicating COX-2 was closely related to tumor angiogenesis further, and may be one of important factors involved in gastric carcinoma angiogenesis. In addition, MVD in the specimens with lymph node metastasis was significantly higher than that without and it was also correlated closely with the depth of tumor invasion,suggesting that tumor angiogenesis in gastric carcinomas might result in cancer cells entering blood circulation, and the lymph node metastasis could be promoted when the gastric cancer cells invade lymphatic vessels. Both COX-2 and MVD were associated with the depth of invasion and lymph-node metastasis, suggesting the effect of COX-2 on angiogenesis can promote metastatic protential as well as tumor invasiveness. Therefore, inducing tumor angiogenesis may be one of mechanisms which COX-2 promotes the development and metastasis of gastric cancer.
In conclusion, COX-2 expression in gastric adenocarcinoma was higher than that in the paracancerous tissues, and was related to lymph node metastasis and the depth of invasion, suggesting COX-2 might correlate with the occurance and advancement of gastric carcinoma; COX-2 expression in gastric carcinoma was closely related to MVD, suggesting COX-2 might be involved in tumor angiogenesis in gastric carcinoma, it is likely that COX-2 inducing angiogenesis may be one of mechanisms which COX-2 promotes the invasion, metastasis of tumor in gastric carcinoma. These findings suggest that COX-2 may be a new therapeutic target for anti-angiogenesis.
Edited by Xu JY
| 1. | Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270:G393-G400. [PubMed] |
| 2. | Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 272] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | DuBois RN, Awad J, Morrow J, Roberts LJ, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J Clin Invest. 1994;93:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97-102. [PubMed] |
| 5. | García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Morgan G. Non-steroidal anti-inflammatory drugs and the chemoprevention of colorectal and oesophageal cancers. Gut. 1996;38:646-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Reeves MJ, Newcomb PA, Trentham-Dietz A, Storer BE, Remington PL. Nonsteroidal anti-inflammatory drug use and protection against colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 1996;5:955-960. [PubMed] |
| 8. | Thun MJ. NSAID use and decreased risk of gastrointestinal cancers. Gastroenterol Clin North Am. 1996;25:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Funkhouser EM, Sharp GB. Aspirin and reduced risk of esophageal carcinoma. Cancer. 1995;76:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Zhuang ZH, Wang LD. NSAID and digestive tract carcinomas. Shijie Huaren Xiaohua Zazhi. 2001;9:1050-1053. |
| 11. | Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1733] [Cited by in RCA: 1678] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 12. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1039] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 13. | Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991-994. [PubMed] |
| 14. | Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987-990. [PubMed] |
| 15. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 16. | Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, Kishimoto Y, Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407-412. [PubMed] |
| 17. | Shirahama T, Sakakura C. Overexpression of cyclooxygenase-2 in squamous cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:558-561. [PubMed] |
| 18. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Wu QM, Li SB, Wang Q, Wang DH, Li XB, Liu CZ. The expres-sion of COX-2 in esophageal carcinoma and its relation to clinicoptahologic characteristics. Shijie Huaren Xiaohua Zazhi. 2001;9:11-14. |
| 20. | Qiu DK, Ma X, Peng YS, Chen XY. Significance of cyclooxygenase-2 expression in human primary hepatocellular carcinoma. World J Gastroenterol. 2002;8:815-817. [PubMed] |
| 21. | Che X, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, Aikou T. Tumor angiogenesis related to growth pattern and lymph node metastasis in early gastric cancer. Chin Med J (Engl). 1998;111:1090-1093. [PubMed] |
| 22. | Shimoyama S, Kaminishi M. Increased angiogenin expression in gastric cancer correlated with cancer progression. J Cancer Res Clin Oncol. 2000;126:468-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yoshikawa T, Yanoma S, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Angiogenesis inhibitor, TNP-470, suppresses growth of peritoneal disseminating foci. Hepatogastroenterology. 2000;47:298-302. [PubMed] |
| 24. | Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, Kuroshima K, Aikou T. Angiogenesis as an unfavorable factor related to lymph node metastasis in early gastric cancer. Ann Surg Oncol. 1998;5:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Maehara Y, Hasuda S, Abe T, Oki E, Kakeji Y, Ohno S, Sugimachi K. Tumor angiogenesis and micrometastasis in bone marrow of patients with early gastric cancer. Clin Cancer Res. 1998;4:2129-2134. [PubMed] |
| 26. | Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1298] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 27. | Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 435] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 28. | Kargman S, Charleson S, Cartwright M, Frank J, Riendeau D, Mancini J, Evans J, O'Neill G. Characterization of Prostaglandin G/H Synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996;111:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 320] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Uefuji K, Ichikura T, Mochizuki H, Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Ratnasinghe D, Tangrea JA, Roth MJ, Dawsey SM, Anver M, Kasprzak BA, Hu N, Wang QH, Taylor PR. Expression of cyclooxygenase-2 in human adenocarcinomas of the gastric cardia and corpus. Oncol Rep. 1999;6:965-968. [PubMed] |
| 31. | Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519-525. [PubMed] |
| 32. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Ohon R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patents with gastric carcinoma. Cancer. 2001;91:1876-1881. [RCA] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 34. | Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:250-253. [PubMed] |
| 35. | Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 1565] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 37. | Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 210] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4848] [Cited by in RCA: 4789] [Article Influence: 165.1] [Reference Citation Analysis (0)] |
| 39. | Masferrer JL, Koki A, Seibert K. COX-2 inhibitors. A new class of antiangiogenic agents. Ann N Y Acad Sci. 1999;889:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-1311. [PubMed] |
| 41. | Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998;17:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |