Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.670
Revised: April 28, 2002
Accepted: May 8, 2002
Published online: April 15, 2003
AIM: To investigate the inhibitory effect of a Chinese herb medicine Astragali radix (AR) on growth of different cancer cell lines.
METHODS: To observe the in vitro effects of AR on tumor cell proliferation by trypan blue exclusion, MTS method and tritium thymidine incorporation assay. Apoptosis was detected by DNA ladder method.
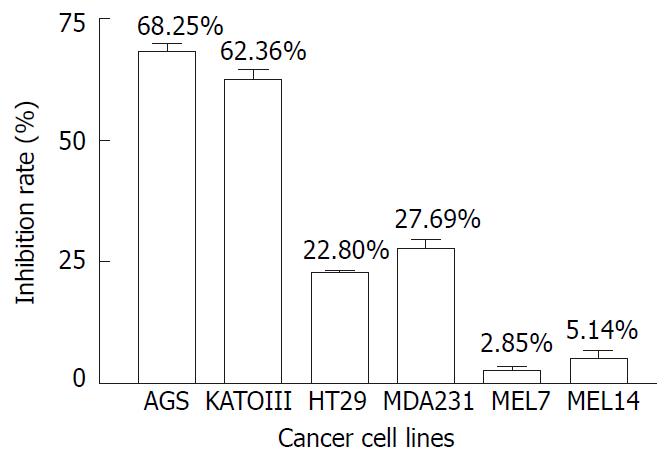
RESULTS: The inhibition rates of AR on the cell respiration of AGS, KATOIII, HT29, MDA231, MEL7 and MEL14 were 68.25%, 62.36%, 22.8%, 27.69%, 2.85% and 5.14% respectively at the concentration of 100 ug/mL; it inhibited AGS DNA synthesis by 87.33% at the concentration of 50 ug/mL. The inhibitory effect on AGS was time-and dose-dependent. AR did not induce apoptosis in AGS cells.
CONCLUSION: AR specifically inhibits gastric cancer cells growth in vitro and the mechanism is mainly cytostatic but not cytotoxic or inducing apoptosis.
- Citation: Lin J, Dong HF, Oppenheim J, Howard O. Effects of astragali radix on the growth of different cancer cell lines. World J Gastroenterol 2003; 9(4): 670-673
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/670.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.670
Astragali radix (AR) is the dried root of Astragalus membranaceus Bge. Var. mongholicus and is used as a tonic in the traditional Chinese medicine. It has been used extensively as an adjuvant[1,2] in cancer treatment and as a phytochemical immune modulator. Kurashige et al[3] reported that AR lowered the incidence of urinary bladder carcinoma in N-butyl-N’-butanolinitrosoamine treated mice by activating the cytotoxicity of lymphocytes and increasing the production of IL-2 and IFN-γ. Lau’s study showed that it also restored the chemiluminescent oxidative burst activity of murine splenic macrophage suppressed by renal cell carcinoma[4]. Wang’s research suggested that an extract of AR had the synergetic effect with IL-2 in activating LAK cells, resulting in reducing the dosage of IL-2 and the associated toxicity[5]. In addition, AR also could promote the proliferation of B cell and the production of immunoglobulin[6] and has a bidirectional modulating effect on T cells. It was reported that it could reduce the suppressive activity of Ts in post-burn mice[7] and also increase Th cell activity in immunodepressed mice[8].
However, there was no report on whether AR affects tumor cells growth directly. In this paper, we studied the effect of an aqueous extract of AR on the growth of different cancer cell lines.
Human gastric cancer cell lines AGS and KATO-III were purchased from the American Type Culture Collection. AGS is a cell line of moderately-poorly differentiated adenocarcinoma and KATO-III is a cell line of signet ring carcinoma. HT29 is a cell line of colon cancer and MDA231 is a breast cancer cell line, both of them were kindly provided by Dr. Bill Murphy. Mel7 and Mel14 are melanoma cell lines, which were gifted by Dr. D. Schadendorf. All the cells were cultured in RPMI1640 medium supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin and Glutamine (GIBCO BRL, Life Technologies, Grand Island, NY, USA) in a humidified atmosphere of 95% air with 5% CO2 at 37 °C as a monolayer.
AR was purchased from Da Xing Chinese herbal medicines store in DC, USA. The aqueous extract was prepared by the Natural Product Branch of National Cancer Institute. 8 grams of aqueous extract was dissolved in 100 ml distilled water and centrifuged at 4000 rpm for 20 min. The supernatant was passed through a 0.22 µm filter (Corning, Costar, NY, USA) and reached a final concentration of 80 mg/mL. The solution was aliquoted and stored at -20 °C for future use.
The herbal treatment was modified from the anti-cancer drug screening program of natural product branch in NCI[9]. Cells were harvested by trypsinization when they were confluenced. 1 × 104 and 3 × 105 cells were seeded in each of the 96-well and 6-well plates respectively and cultured in RPMI1640 medium supplemented with 5% FBS for 24 hours. Then different concentrations of AR were added in each well to attain a series of different concentrations (1, 12.5, 25, 50 and 100 µg/mL) and incubated for 48 hours for further measurements.
Cell growth was measured by MTS assay. The Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay Kit was purchased from Promega, WI, USA. Briefly, MTS and PMS were mixed at the ratio of 20:1 immediately before being added to the samples. 20 µl of MTS/PMS solution was added to each of the 96-well plate and incubated at 37 °C in a humidified 5% CO2 atmosphere for 3 hours. The absorbance was read at 490 nm using Bio-Tek’s Power Wave x reader-assay system (BIO-TEK Instruments INC, VT, USA). Each sample was triplicated.
Cell DNA synthesis was measured by 3H-thymidine incorporation assay. At the end of herbal treatment, 1 µCi of 3H-thymidine was added in each well of a 96-well plate. Then the plate was incubated at 37 °C in a humidified 5% CO2 atmosphere for 4 hours and stored at -70 °C overnight. On the second day, the frozen plate was thawed at 37 °C and the DNA was transferred to the filtermat using multi-channel cell collector. The filtermat was washed with distilled water thrice and 95% alcohol once. 4.5 ml Betaplate Scint was added to the filtermat and read the filtermat with liquid scintillation and luminescence counter (Perkin Elmer Wallac Inc. MD, USA). Each sample was quadruplicated.
Cells were cultured and treated in 6-well plate. At the end of the treatment, the cells were collected by trypsinization and counted with 1% Trypan-blue. Each sample was triplicated.
DNA ladder was measured as described previously[10] with some modification. Briefly, 1 × 106 cells in 0.5 ml were lysed in 1 ml DNA lysis buffer containing 1 M Tris-HCl, 0.2 M EDTA, 4 M NaCl, 20% SDS and 400 µg/mL proteinase K) and incubated at 37 °C overnight. DNA was extracted in Phase LockGel eppendorf (Eppendorf Scientific Inc. NY, USA) with an equal volume of phenol/choloroform/isoamyl alcohol (25:24:1) and precipitated with 2 volumes of ice-cold 100% ethanol and 1/10 volume of 3 M sodium acetate at -70 °C for 1 hr. DNA was collected, washed with ice-cold 70% ethanol once and dried in air. Then DNA was dissolved in TE buffer containing 10 µg/mL RNase I and incubated at 37 °C for 1 hr. Equal amounts of DNA (10 µg/well) were electrophoresed in 1.8% agarose gels impregnated with ethidium bromide (0.1 µg/mL) for 2 hr at 70 V. DNA fragments were visualized by UV transillumination.
The inhibition rate was calculated by the following formula: inhibition rate (%) = (OD/CPMcontrol-OD/CPMtest)/OD/CPMcontrol × 100%. Student’s t-test was used to compare the results. All P values were described by two-tailed analysis. P < 0.05 was considered statistically significant.
Figure 1 illustrated the inhibiting effects of AR on the proliferation of 6 tumor cell lines, including AGS, KATOIII, HT29, MDA231, MEL7 and MEL14, measured by MTS method. It was found that after 48 hours incubation, 100 µg/mL of AR had greater inhibiting effect on gastric tumor cell lines of AGS and KATOIII than the other cancer cell lines. It inhibited the growth of AGS and KATOIII by 68.25% and 62.36% respectively, compared to only 22.8%, 27.69%, 2.85% and 5.14% reduction in cell growth of HT29, MDA231, MEL7 and MEL14. It appeared to be selectively inhibiting the growth of gastric cancer cell line.
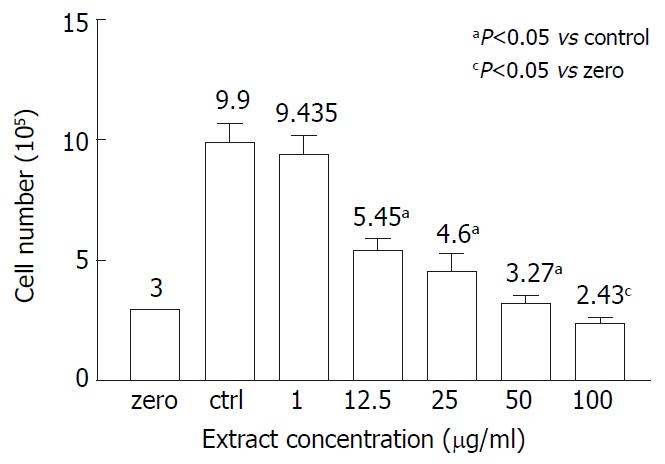
Whether the inhibitory effect of AR on AGS growth was cytotoxic was determined by the trypan blue measurement. Figure 2 showed the viable cell numbers of AGS treated with different concentrations of AR. In the range from 1 to 50 µg/mL, AR had a dose-dependent inhibiting effect on AGS growth and almost completely inhibited the AGS growth at the concentration of 50 µg/mL, of which the cell number of the test group was very close to the cell number seeded at the beginning of the test and few cells were stained blue. However, at the concentration of 100 µg/mL, AR showed cytotoxicity on AGS, of which the cell number was lower than the initial cell number.
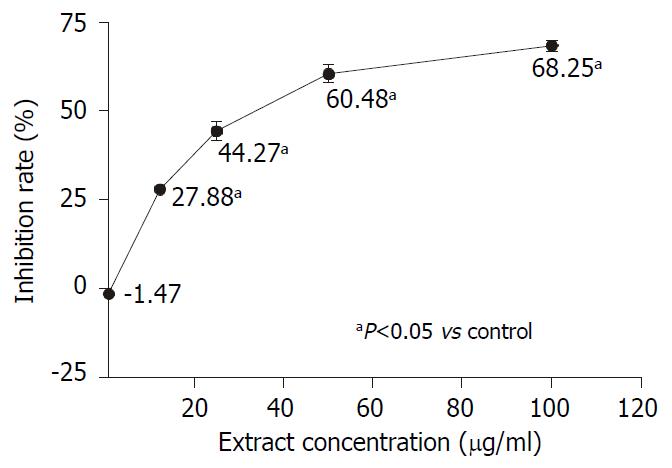
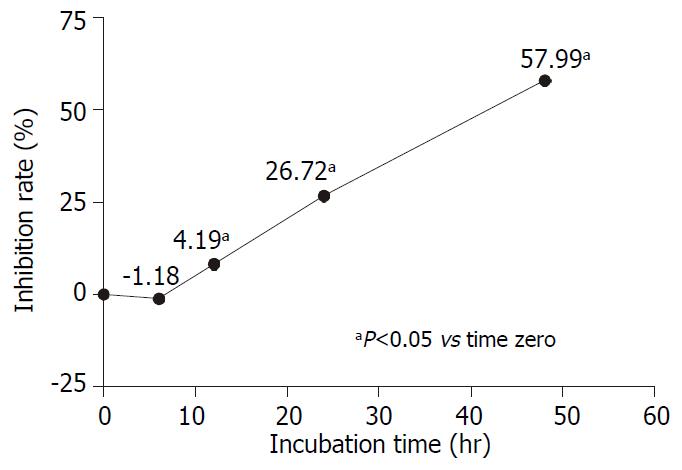
The dose-dependent and time-dependent effects of AR on AGS growth were observed by MTS assay. AGS cells were treated with different concentrations of AR for 48 hours. Figure 3 showed that the inhibitory effect of AR on AGS was proportional to the extract concentrations. In the time-course experiment, AGS cells were treated with 50 µg/mL of AR and incubated for 6, 12, 24 and 48 hours respectively. In Figure 4, it was found that AR began to inhibit the cell growth significantly at the 12th hour and the effect was also improved linearly while prolonging the incubation time.
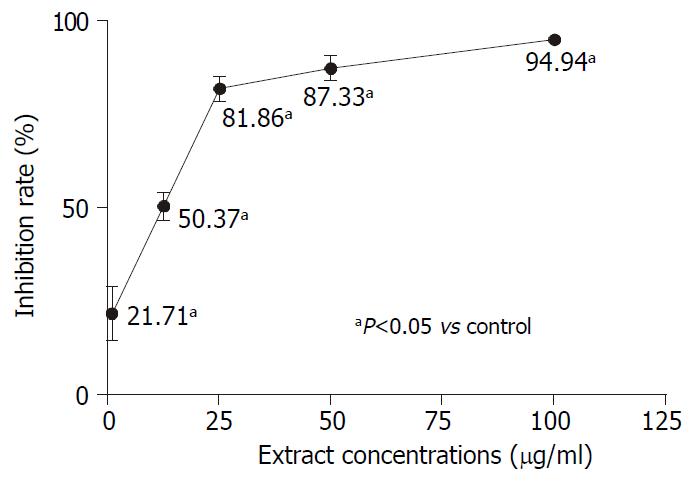
Tritium-labeled thymidine incorporation assay was used to evaluate the effect of AR on AGS DNA synthesis. AGS cells were treated with different concentrations of AR for 48 hours. Figure 5 demonstrated that AR could significantly inhibit the DNA synthesis of AGS. It was also observed that the inhibiting effect of AR increased sharply from the concentrations of 1 µg/mL to 25 µg/mL and it increased slowly from then on.
DNA fragmentation method was used to observe whether apoptosis was induced in AGS by AR. AGS cells were treated with different concentrations of AR for 48 hours. However, there was no DNA ladder appeared in the electropheresis gel. So the herbal extract had no effect on AGS apoptosis.
AR is a tonic herbal medicine which is used as an adjuvant extensively in the treatment of various cancers, such as lung cancer, digestive tract cancers, renal cancer, bladder cancer[1,2,11], melanoma and AIDS[12]. It had been reported that it could improve the host immune function suppressed by tumors and relieve the marrow suppression induced by chemotherapeutic agents so that it could make the patients more tolerant to chemotherapeutic therapy. Although there were some studies showing that AR could inhibit the tumor growth in vivo, the anti-cancer effect was testified through activating the activity of Th, LAK and macrophages and promoting the production of IL-2 and IFN-γ. However, whether it has direct inhibition effect on tumors cells has not been elucidated yet.
In this study, 6 different cancer cell lines were used to test the inhibitory effect of AR. After 48 hours incubation, AR inhibited the gastric cancer cell lines of AGS and KATOIII growth by 68.25% and 62.36%, compared to the inhibition rates of colon cancer cell line HT29, breast cancer cell line MDA231 and melanoma cell lines of Mel7 and Mel14 which were 22.8%, 27.69%, 2.85% and 5.14% respectively, suggesting that AR has anti-cancer effect on gastric cancer cell lines. To fully confirm this, many other kinds of cancer cell lines should be tested in order to determine the specificity of the effect. AGS and KATOIII are two different gastric cancer cell lines. AGS is a moderately-poorly differentiated adenocarcinoma cell line while KATOIII is a cell line of signet ring cell carcinoma, but AR has almost the same inhibitory effect on both cell lines.
Many herbal extracts can inhibit cell growth in vitro through their cytotoxic effect[13-16]. We determined whether the inhibitory effect of AR was due to cytotoxicitiy. In order to clarify this question, we measured the cell growth with trypan blue exclusion method. The initial cell number was 300000/well and in the untreated wells the cell number reached 990000/well after 48 hours incubation. The cell number in testing group treated with 1 µg/mL of AR was 943500/well (P > 0.05, vs control group). However, the cell numbers of the other testing groups treated with AR from the concentrations of 12.5 µg/mL to 100 µg/mL were significantly lower than that of the control group and but higher than the initial cell number except for the testing group treated with 100 µg/mL AR, whose cell number was only 243000/well (P = 0.017, compared to the initial cell number). Few cells stained trypan blue could be seen in the testing groups treated with AR at the concentration lower than 100 µg/mL. So the inhibition effect of AR at the concentrations of 50 µg/mL or below was not cytotoxic while AR is cytotoxic to AGS cell at the concentration of 100 µg/mL. Further studies showed that the inhibition effect of AR on AGS was dose- and time-dependent. The maximal concentration without cytotoxicity was around 50 µg/mL and the corresponding inhibition rate was 60.48%. AR came into effect at the 12th hour and reached its peak at the 48th hour.
One characteristic of the tumor cells is that their rapid proliferations are accompanied by DNA synthesis and cellular replication. Tritium labeled thymidine incorporation assay reflects the cellular DNA synthesis. Our results showed that the DNA synthesis of AGS was significantly inhibited by AR. The inhibition rate increased sharply from 21.71% to 81.86% at the concentrations ranging from 1 µg/mL to 25 µg/mL, then it reached a plateau. So AR might suppress AGS cell growth through inhibition of DNA synthesis. The inhibition rates of AGS cell growth expressed by MTS method and tritium labeled thymidine incorporation assay were different. The latter was higher than the former. This maybe attributed to that MTS and tritium labeled thymidine incorporation assay were two different indirect measurements used in cell growth evaluation. MTS reflects the cell growth at the level of cell respiration while tritium labeled thymidine incorporation assay at the level of DNA synthesis. When the cellular DNA synthesis is suppressed, the cells might be in an inactive status in which the cells could still respire. So the inhibition rate of cell growth expressed by MTS is lower than that expressed by tritium labeled thymidine incorporation assay.
Apoptosis is a kind of programming cell death whose impairment may induce cell immortality and carcinogenesis[17]. Some agents such as aspirin, indomethacin and arsenic trioxide[10,18] and some herbs such as Anemarrhena asphodeloides Bunge, Albizzia Lucidior I. Nielsen and Paeoniae radix[19-21] were reported to have the ability of inducing apoptosis in gastric cancer cells and inhibiting their growth. However, apoptosis could not be observed in AGS cells treated with AR. Combined with the results of trypan blue experiment that there were few cells, which were treated with AR, stained blue, the inhibition effect of AR on AGS is not through the mechanism of cytotoxic or apoptosis.
The aqueous and organic extracts of AR were used in this study. But the organic extract had no effect on AGS growth (data not shown). So the effective anti-cancer components are in the aqueous extract. Polysaccharide[22], astragaloside[23], and flavonoids[24] have been found in AR. Some studies showed that the polysaccharides and flavonoids from other herbs had the anti-tumor efficiency in vitro and in vivo[25-29]. But up to now, there has no evidence showing that these compounds from AR have anti-cancer activity in vitro. Hence, further studies are required. Selenium toxicity has been confirmed in the livestock consuming plants of the genus Astragalus and selenium was reported to have the carcinostatic activity in the animals[30,31]. So selenium might play a critical role in AR inhibiting AGS growth.
In conclusion, the inhibitory effect of AR on the growth of gastric cancer cell line of AGS is mainly cytostatic.
Edited by Wu XN
| 1. | Cha RJ, Zeng DW, Chang QS. [Non-surgical treatment of small cell lung cancer with chemo-radio-immunotherapy and traditional Chinese medicine]. Zhonghua Neike Zazhi. 1994;33:462-466. [PubMed] |
| 2. | Li NQ. [Clinical and experimental study on shen-qi injection with chemotherapy in the treatment of malignant tumor of digestive tract]. Zhongguo Zhongxiyi Jiehe Zazhi. 1992;12:588-592, 579. [PubMed] |
| 3. | Kurashige S, Akuzawa Y, Endo F. Effects of astragali radix extract on carcinogenesis, cytokine production, and cytotoxicity in mice treated with a carcinogen, N-butyl-N'-butanolnitrosoamine. Cancer Invest. 1999;17:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lau BH, Ruckle HC, Botolazzo T, Lui PD. Chinese medicinal herbs inhibit growth of murine renal cell carcinoma. Cancer Biother. 1994;9:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Qian XJ, Hadley HR, Lau BH. Phytochemicals potentiate interleukin-2 generated lymphokine-activated killer cell cytotoxicity against murine renal cell carcinoma. Mol Biother. 1992;4:143-146. [PubMed] |
| 6. | Yoshida Y, Wang MQ, Liu JN, Shan BE, Yamashita U. Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol. 1997;19:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Liang H, Zhang Y, Geng B. [The effect of astragalus polysaccharides (APS) on cell mediated immunity (CMI) in burned mice]. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1994;10:138-141. [PubMed] |
| 8. | Zhao KS, Mancini C, Doria G. Enhancement of the immune response in mice by Astragalus membranaceus extracts. Immunopharmacology. 1990;20:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589-601. [PubMed] |
| 10. | Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho CH, Lai KC, Lin MC, Kung HF, Lam SK. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer. 2001;91:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Rittenhouse JR, Lui PD, Lau BH. Chinese medicinal herbs reverse macrophage suppression induced by urological tumors. J Urol. 1991;146:486-490. [PubMed] |
| 12. | Chu DT, Lin JR, Wong W. [The in vitro potentiation of LAK cell cytotoxicity in cancer and aids patients induced by F3--a fractionated extract of Astragalus membranaceus]. Zhonghua Zhongliu Zazhi. 1994;16:167-171. [PubMed] |
| 13. | Chen SB, Gao GY, Li YS, Yu SC, Xiao PG. Cytotoxic constituents from Aquilegia ecalcarata. Planta Med. 2002;68:554-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Wang S, Zhang YJ, Chen RY, Yu DQ. Goniolactones A-F, six new styrylpyrone derivatives from the roots of Goniothalamus cheliensis. J Nat Prod. 2002;65:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Fan CQ, Sun HF, Chen SN, Yue JM, Lin ZW, Sun HD. Triterpene saponins from Craniotome furcata. Nat Prod Lett. 2002;16:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Li J, Liang N, Mo L, Zhang X, He C. [Comparison of the cytotoxicity of five constituents from Pteris semipinnata L. in vitro and the analysis of their structure-activity relationships]. Yaoxue Xuebao. 1998;33:641-644. [PubMed] |
| 17. | Wyllie AH, Bellamy CO, Bubb VJ, Clarke AR, Corbet S, Curtis L, Harrison DJ, Hooper ML, Toft N, Webb S. Apoptosis and carcinogenesis. Br J Cancer. 1999;80 Suppl 1:34-37. [PubMed] |
| 18. | Zhou XM, Wong BC, Fan XM, Zhang HB, Lin MC, Kung HF, Fan DM, Lam SK. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis. 2001;22:1393-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Takeda Y, Togashi H, Matsuo T, Shinzawa H, Takeda Y, Takahashi T. Growth inhibition and apoptosis of gastric cancer cell lines by Anemarrhena asphodeloides Bunge. J Gastroenterol. 2001;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Cai B, Zhang HF, Zhang DY, Cui CB, Li WX. [Apoptosis-inducing activity of extract from Chinese herb, Albizzia Lucidior I. Nielsen]. Aizheng. 2002;21:373-378. [PubMed] |
| 21. | Lee SM, Li ML, Tse YC, Leung SC, Lee MM, Tsui SK, Fung KP, Lee CY, Waye MM. Paeoniae Radix, a Chinese herbal extract, inhibit hepatoma cells growth by inducing apoptosis in a p53 independent pathway. Life Sci. 2002;71:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Zheng Z, Liu D, Song C, Cheng C, Hu Z. Studies on chemical constituents and immunological function activity of hairy root of Astragalus membranaceus. Chin J Biotechnol. 1998;14:93-97. [PubMed] |
| 23. | Hirotani M, Zhou Y, Rui H, Furuya T. Cycloartane triterpene glycosides from the hairy root cultures of Astragalus membranaceus. Phytochemistry. 1994;37:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Lin LZ, He XG, Lindenmaier M, Nolan G, Yang J, Cleary M, Qiu SX, Cordell GA. Liquid chromatography-electrospray ionization mass spectrometry study of the flavonoids of the roots of Astragalus mongholicus and A. membranaceus. J Chromatogr A. 2000;876:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Lu X, Su M, Li Y, Zeng L, Liu X, Li J, Zheng B, Wang S. Effect of Acanthopanax giraldii Harms Var. Hispidus Hoo polysaccharides on the human gastric cancer cell line SGC-7901 and its possible mechanism. Chin Med J (Engl). 2002;115:716-721. [PubMed] |
| 26. | Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, Ho CK. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Wang HB, Zheng QY. Effects of Phytolacca acinosa polysaccharides I with different schedules on its antitumor efficiency in tumor bearing mice and production of IL-1, IL-2, IL-6, TNF, CSF activity in normal mice. Immunopharmacol Immunotoxicol. 1997;19:197-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Chang WH, Chen CH, Lu FJ. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002;68:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Zheng GQ. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. 1994;60:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med. 1994;17:45-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 395] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Harrison PR, Lanfear J, Wu L, Fleming J, McGarry L, Blower L. Chemopreventive and growth inhibitory effects of selenium. Biomed Environ Sci. 1997;10:235-245. [PubMed] |