Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.562
Revised: February 23, 2002
Accepted: March 5, 2002
Published online: March 15, 2003
AIM: To study the role of mitochondrial dysfunction in hydrogen peroxide-induced apoptosis of intestinal epithelial cells.
METHODS: Hydrogen peroxide-induced apoptosis of human intestinal epithelial cell line SW-480 was established. Cell apoptosis was determined by Annexin-V and PI double-stained flow cytometry and DNA gel electrophoresis. Morphological changes were examined with light and electron microscopy. For other observations, mitochondrial function, cytochrome c release, mitochondrial translocation and membrane potential were determined simultaneously.
RESULTS: Percentage of apoptotic cells induced with 400 μmol/L hydrogen peroxide increased significantly at l h or 3 h after stimulation and recovered rapidly. Meanwhile percentage of apoptotic cells induced with 4 mmol/L hydrogen peroxide increased with time. In accordance with these changes, we observed decreased mitochondrial function in 400 μmol/L H2O2-stimualted cells at 1 h or 3 h and in 4 mmol/L H2O2-stimualted cells at times examined. Correspondingly, swelling cristae and vacuole-like mitochondria were noted. Release of cytochrome c, decreased mitochondrial membrane potential and mitochondrial translocation were also found to be the early signs of apoptosis.
CONCLUSION: Dysfunctional mitochondria play a role in the apoptosis of SW-480 cell line induced by hydrogen peroxide.
- Citation: Li JM, Zhou H, Cai Q, Xiao GX. Role of mitochondrial dysfunction in hydrogen peroxide-induced apoptosis of intestinal epithelial cells. World J Gastroenterol 2003; 9(3): 562-567
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.562
Hidden injuries of gut during the early stage of severe burn may contribute to early translocation of bacteria or its endotoxin. Although the mechanisms of gut barrier dysfunction postburn are unclear[1-10], evidences recently indicate that apoptosis of intestinal epithelial cells after thermal injury may be one of possible factors leading to gut barrier dysfunction[11,12]. Apoptosis of intestinal epithelial cells induced by excessive reactive oxygen species released by activated polymorphonuclear leukocytes and vascular endothelia cells plays a role in the pathogenesis of dysfunction of intestinal mucosa. Besides, the role of mitochondrion in the development of apoptosis has been emphasized recently[13]. We have found that differential expression of mitochondrial genes encoding cytochrome c oxidase and ATPase was involved in apoptosis of intestinal epithelial cells by affecting activities of cytochorme c oxidase and ATPase[14]. So mitochondrial dysfunction may contribute to the apoptosis of intestinal epithelial cells. In the present study, possible relationship between mitochondrial dysfunction and apoptosis was studied in hydrogen peroxide-induced apoptosis model of SW-480 cells.
Human intestinal epithelial cell line SW-480 stored routinely in our laboratory was cultured in RPMI1640 supplemented with 10%(V/V) heat-inactivated newborn calf serum (Hyclone), 100 units/ml of penicillin, 0.1 mg/ml streptomycin and 2 mM l-glutamine at 37 °C in a humidified 5% CO2 incubator. Confluent cells were prepared for further studies.
Experimental cells were treated with 4 mmol/L or 400 μmol/L hydrogen peroxide. Cells without stimulation by hydrogen peroxide were prepared as control.
Samples were fixed, embedded and sectioned routinely. Ultrastructural changes of mitochondria were observed with transmission electron microscopy.
Annexin-V and PI double staining kit (Roche) was used to assess apoptosis in hydrogen peroxide-stimulated SW-480 cells. Ten thousands of cells were counted, and data acquisition and analysis were performed in a Becton Dickinson FACS-can flow cytometer using the CellQuest software.
The DNA fragmentation pattern (DNA ladder) was demonstrated by agarose gel electrophoresis described previously[15].
Cells were grown on glass cover slips. After treated with 4 mmol/L or 400 μmol/L hydrogen peroxide, samples were fixed in 10% neutral formaldehyde solution for 30 min with PBS rinsing for several times. Then, cells were stained by overnight incubation with 100-fold diluted rabbit anti-human cytochrome c polyclonal antibody (Oncogene) at 4 °C, followed by extensive washing with phosphate-buffered saline and a 30 min incubation with biotin-binding goat anti-rabbit antibody. After another 30 min incubation with horseradish peroxides conjugated ovalbumin, the specimens were colorized and photographed.
Mitochondrial function was assessed by MTT (3, (4,5-dimtthylthiazol- 2-yl) 2, 5-diphenyltetrazolium bromide) assay as described previously[16]. Cells were cultured in 96-well plates 5000 cells for each well at 37 °C in a humidified 5% CO2 incubator. Confluent cells were prepared for further studies. After treated with hydrogen peroxides and washed with phosphate-buffered saline, cells were incubated with MTT (2 μg/ml, Sigma) and RPMI1640 medium without serum at 37 °C for 1 h and dissolved with dimethyl sulphoxide. The absorbance at 570nm, which represented the total mitochondrial function, was recorded.
Cells were grown on glass cover slips. After treated with 4 mmol/L or 400 μmol/L hydrogen peroxide, cells were incubated with Rhodamine 123 (1 μmol/L, Molecular probe) and RPMI1640 medium without serum at 37 °C for 30 min, fluorescence intensity was determined by confocal microscope (Leica) with fixed parameters, cells in three random-selected visual fields from each group were scanned and analysised.
As described previously, cells were seeded in chambered cover slips and preincubated overnight at 37 °C in a humidified 5% CO2 air incubator. After the cells were treated with hydrogen peroxide, mitochondria were stained with Rhodamine 123 for 30 min at 37 °C before analysis. The distribution of mitochondria was analyzed with a Zeiss TSTN confocal microscope[17].
Data were summarized as mean ± SD. Student’s t test was used for multiple comparisons between groups. P values less than 0.05 were considered to be statistically significant.
Percentage of apoptotic cells induced with 400 μmol/L hydrogen peroxide increased significantly at l h or 3 h after stimulation and recovered rapidly. Meanwhile percentage of apoptotic cells induced with 4 mmol/L hydrogen peroxide increased with the time, which indicated that the irreversible changes had taken place (Table 1)
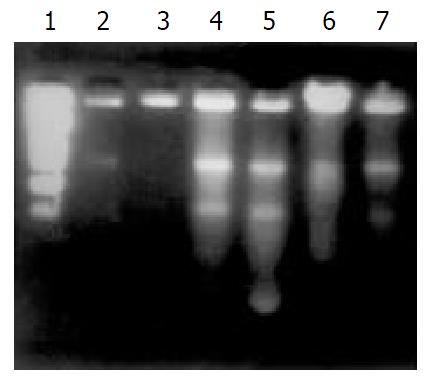
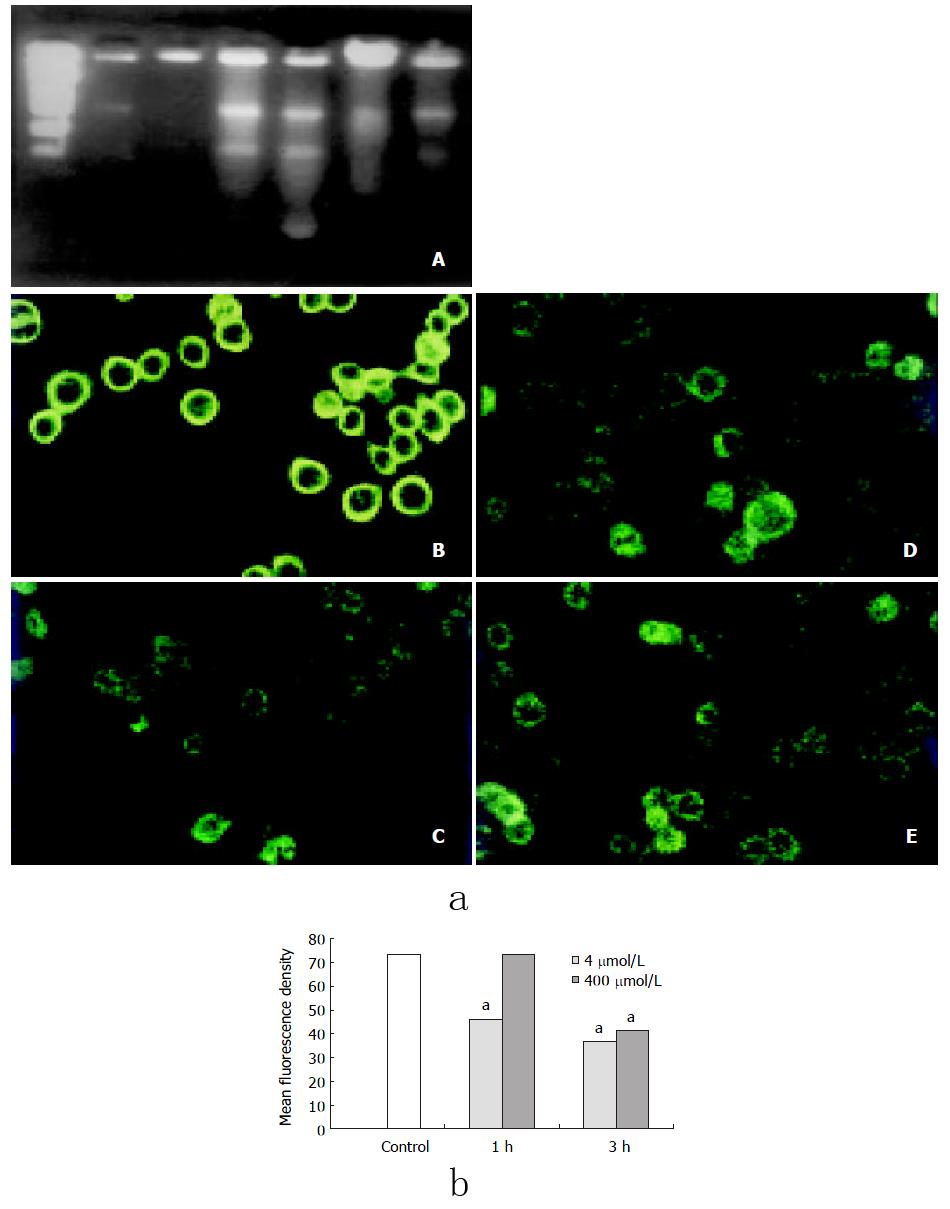
DNA ladder in both 4 mmol/L and 400 μmol/L H2O2-stimualted groups were clearly observed by DNA fragmentation assay at 1h or 3h after stimulation (Figure 1).
The MTT assay is based on the conversion of MTT (light yellow) to formazan (blue) by the mitochondrial enzyme succinate dehydrogenase and has been widely used as an indicator of cellular respiration and viability[3]. We observed decreased mitochondrial function in 400 μmol/L H2O2-stimualted cells at 1 h or 3 h after stimulation and in 4 mmol/ L H2O2-stimualted cells at times examined (Table 2). Interestingly, these changes were in accordance with the apoptosis induced by hydrogen peroxide.
| Group | 1 h | 3 h | 6 h | 12 h | 24 h |
| Control | 1.971 ± 0.101 | 1.996 ± 0.013 | 1.867 ± 0.008 | 2.087 ± 0.126 | 2.189 ± 0.178 |
| 4mmol/L | 0.864 ± 0.116a | 0.756 ± 0.023a | 0.612 ± 0.006a | 0.518 ± 0.035a | 0.373 ± 0.043a |
| 400mmol/L | 1.588 ± 0.005 | 1.277 ± 0.300a | 1.778 ± 0.098 | 1.599 ± 0.214 | 1.899 ± 0.031 |
| 200mmol/L | 1.626 ± 0.262 | 1.914 ± 0.046 | 1.941 ± 0.032 | 1.787 ± 0.033 | 1.962 ± 0.149 |
| 100mmol/L | 1.683 ± 0.070 | 1.973 ± 0.048 | 1.933 ± 0.094 | 1.901 ± 0.097 | 2.079 ± 0.081 |
| 50mmol/L | 1.865 ± 0.122 | 1.974 ± 0.080 | 2.077 ± 0.077 | 1.876 ± 0.053 | 1.922 ± 0.048 |
Swelling cristae and vacuole-like mitochondria were found in hydrogen peroxide -treated cells.
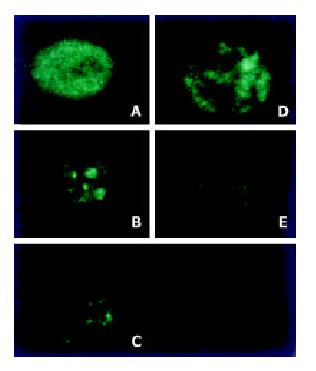
Mitochondria were observed to be evolved from an originally scattered, bipolar and nearly symmetric distribution to the asymmetric clustered state in the majority of cells treated with H2O2 (Figure 2).
Decreased mitochondrial membrane potential was observed in cells at 3 h after 400 μmol/L H2O2 stimulation and at 1h after 4 mmol/L H2O2 treatment (Figure 3).
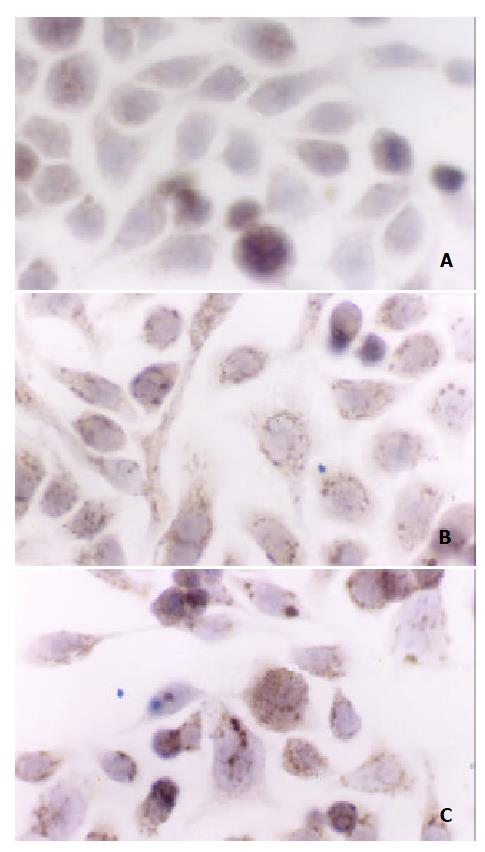
Cytochrome c release could be found in both 400mmol/L and 4mmol/L H2O2-stimualted cells 30 min after stimulation by immunochemistry assay (Figure 4).
Metabolism of intestinal mucosal epithelium is so active that it is very sensitive to changes of energy supplies in normal conditions[11]. Many researches have demonstrated that gut is a sensitive organ to be injured postburn[18-22]. Intestinal mucosal injury can be caused by excessive reactive oxygen species (ROS) released by polymorphonuclear leukocytes and vascular endothelial cells, which is also involved in translocation of intestinal bacteria and its endotoxin.
Our results showed that hydrogen peroxide could lead to injures of intestinal epithelial cells in the concentration of both 400 μmol/L and 4 mmol/L. From the results of DNA ladders and flow cytometry, apoptosis could be considered one of the main mechanisms for the injury. This indicated that the in vitro model of hydrogen peroxide-stimulated SW-480 cells used in the present study could be used to investigate mitochndrial dysfunction in apoptosis of intestinal epithelial cells, and it also might be helpful to study the role of mitochondria in ROS-induced injuries of intestinal epithelial cells and to clarify mechanism of gut barrier dysfunction.
Role of mitochondria in the pathogenesis of apoptosis has been well defined[23-27]. Mitochondria, a kind of organelle controlling growing, breeding and dying of eukaryocyte, perform their functions by production of ATP, production of ROS, ROS are also known as signals regulating gene expression and triggering of cell death[28,29]. Many stimulators like ROS, Ca2+ and cytokines could activate caspases by inducing cytochrome c release.
Our results showed that the apoptotic cells were characterized with swelling or vacuole-like mitochondria. It was considered previously that the apoptotic cells manifested condensed chromatin but intact mitochondria. Now much more evidences found that significant changes of mitochondria such as swelling, megamitochondria[30], mitochodrial pyknosis and disrupted out-membrane have been taken place in many apoptosis models[31]. Mitochodrial pyknosis was characterized with decreased size and condensed matrix of mitochondria. In apoptotic model of sympathetic neuron triggered by nerve growth factor (NGF) deprivation, the transition from normal to condensed morphology could be reversible following readdition of NGF to the neuron culture[25]. In addition, the mitochondrial distribution within cells was profoundly affected during apoptosis. Mitochondria were normally dispersed throughout the entire cell. However, during the apoptosis triggered by tumor necrosis factor (TNF-α) a perinuclear clustering of mitochondria could be observed. Both mitochondrial condensation and perinuclear clustering occurred following production of the Bcl-2-related proapoptotic protein Bax in many cell types[25].
Evidence showed that the spatial distribution of mitochondria evolved from an originally scattered, bipolar or nearly symmetric distribution to an asymmetric, clustered distribution in the majority of the cells within 1 h of treatment of L929 cells with TNF[17]. Study also indicated that hydrogen peroxide could not lead to the mitochondrial translocation as TNF did[17]. Interestingly, we found that hydrogen peroxide could induce mitochondrial translocation and massive aggregation by confocal microscopy and 3-D reconstruction technique, which was accompanied by decrease of mitochondrial membrane potential. So our results suggested that mitochondrial translocation may play a role in reactive oxygen species (ROS)-induced injuries of intestinal epithelial cells. As some researches proposed, the condensation of mitochondria may play roles in the inducing of cytochrome c release, generating high ATP levels in energy dependent apoptotic events and facilitating the translocation of mitochondrial proteins to the nucleus. Its mechanism is still uncertain.
The mitochondrial transmembrane potential has been found to be decreased in many apoptosis models, which indicates the opening of a large conductance channel known as the mitochondrial PT pore[32-36]. PT pore opening results in a volume dysregulation of mitochondria due to the hyperosmolality of the matrix, which causes the matrix space to be expanded. Because the mitochondrial inner membrane with its folded cristae possesses a larger surface area than the outer membrane, this matrix volume expansion can eventually cause the outer membrane rupture, releasing caspase-activating proteins located within the intermembrane space into the cytosol.
We observed that hydrogen peroxide could result in the collapse of mitochondrial membrane potential, if we related it with the release of cytochrome c, we may get the conclusion that hydrogen peroxide caused the release of cytochrome c from mitochondria to cytosol followed by the increased permeability mitochondrial membrane and the opening of mitochondrial PT pore, which initiated cascade reaction of apoptosis events. This idea has been confirmed by some studies[37,38].
Recent progress in studies on apoptosis has revealed that cytochrome c is a pro-apoptotic factor[39]. It is released from its places on the outer surface of the inner mitochondrial membrane at early steps of apoptosis and, combining with some cytosolic proteins, activates conversion of the latent apoptosis-promoting protease pro-caspase-9 to its active form[39]. Our results also indicated that cytochorme c was released early in hydrogen peroxide-stimulated SW-480 cells.
Our results found that the morphological and functional changes of mitochondria appeared in SW-480 cells treated with hydrogen peroxide and correlated with development of cell apoptosis. Decreased mitochondrial membrane potential or early release of cytochrome c would be the early signs of apoptosis, which suggested mitochondrial dysfunction might be the key event in the development of apoptosis. We also observed mitochondrial translocation, which was reported in TNF-stimulated L929 cells but not in hydrogen peroxide-stimulated cells[33]. Mitochondrial translocation suggested that cytoskeleton be involved in apoptosis induced by hydrogen peroxide.
Many researches indicated that oxidative stress led to mutation of mitochondrial genes[40-50]. Researches showed that there are some links between mitochondrial dysfunction and injuries of mitochondrial DNA or abnormal expression of mitochondrial genes in hydrogen peroxide-stimulated vascular endothelial cells and smooth muscle cells[50]. Our results also indicated that mitochondrial genes were involved in apoptosis of SW-480 cells. Injuries of mitochondrial genes may contribute to early mitochondrial dysfunction. Relationship between response of mitochondrial genes and dysfunctional mitochondria would be the next problem to be answered.
Edited by Zhu L
| 1. | Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836-840. [PubMed] |
| 2. | Meng AH, Ling YL, Zhang XP, Zhao XY, Zhang JL. CCK-8 inhibits expression of TNF-alpha in the spleen of endotoxic shock rats and signal transduction mechanism of p38 MAPK. World J Gastroenterol. 2002;8:139-143. [PubMed] |
| 3. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Relationship between cytokine mRNA expression and organ damage following cecal ligation and puncture. World J Gastroenterol. 2002;8:131-134. [PubMed] |
| 4. | Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol. 2002;8:124-127. [PubMed] |
| 5. | Yu PW, Xiao GX, Qin Xj LX, Wang ZQ. The effects of PAF antagonist on intestinal mucosal microcirculation after burn in rats. World J Gastroenterol. 2000;6:906-908. [PubMed] |
| 6. | Qin RY, Zou SQ, Wu ZD, Qiu FZ. Influence of splanchnic vascular infusion on the content of endotoxins in plasma and the translocation of intestinal bacteria in rats with acute hemorrhage necrosis pancreatitis. World J Gastroenterol. 2000;6:577-580. [PubMed] |
| 7. | Wang QG, He LY, Chen YW, Hu SL. Enzymohistochemical study on burn effect on rat intestinal NOS. World J Gastroenterol. 2000;6:421-423. [PubMed] |
| 8. | Fu XB, Yang YH, Sun TZ, Gu XM, Jiang LX, Sun XQ, Sheng ZY. Effect of intestinal ischemia-reperfusion on expressions of endogenous basic fibroblast growth factor and transforming growth factor betain lung and its relation with lung repair. World J Gastroenterol. 2000;6:353-355. [PubMed] |
| 9. | Fu WL, Xiao GX, Yue XL, Hua C, Lei MP. Tracing method study of bacterial translocation in vivo. World J Gastroenterol. 2000;6:153-155. [PubMed] |
| 10. | Yang YH, Fu XB, Sun TZ, Jiang LX, Gu XM. bFGF and TGFbeta expression in rat kidneys after ischemic/ reperfusional gut injury and its relationship with tissue repair. World J Gastroenterol. 2000;6:147-149. [PubMed] |
| 11. | Ramzy PI, Wolf SE, Irtun O, Hart DW, Thompson JC, Herndon DN. Gut epithelial apoptosis after severe burn: effects of gut hypoperfusion. J Am Coll Surg. 2000;190:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Wolf SE, Ikeda H, Matin S, Debroy MA, Rajaraman S, Herndon DN, Thompson JC. Cutaneous burn increases apoptosis in the gut epithelium of mice. J Am Coll Surg. 1999;188:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Li JM, Cai Q, Zhou H, Xiao GX. Effects of hydrogen peroxide on mitochondrial gene expression of intestinal epithelial cells. World J Gastroenterol. 2002;8:1117-1122. [PubMed] |
| 15. | Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, Kaufman RJ, Kitajima S. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J Biol Chem. 2001;276:35867-35874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Salazar JJ, Van Houten B. Preferential mitochondrial DNA injury caused by glucose oxidase as a steady generator of hydrogen peroxide in human fibroblasts. Mutat Res. 1997;385:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | De Vos K, Goossens V, Boone E, Vercammen D, Vancompernolle K, Vandenabeele P, Haegeman G, Fiers W, Grooten J. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J Biol Chem. 1998;273:9673-9680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Cone JB, Wallace BH, Lubansky HJ, Caldwell FT. Manipulation of the inflammatory response to burn injury. J Trauma. 1997;43:41-45; discussion 45-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Dries DJ, Lorenz K, Kovacs EJ. Differential neutrophil traffic in gut and lung after scald injury. J Burn Care Rehabil. 2001;22:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Jeschke MG, Debroy MA, Wolf SE, Rajaraman S, Thompson JC. Burn and starvation increase programmed cell death in small bowel epithelial cells. Dig Dis Sci. 2000;45:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Eaves-Pyles T, Alexander JW. Rapid and prolonged impairment of gut barrier function after thermal injury in mice. Shock. 1998;9:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Baskaran H, Yarmush ML, Berthiaume F. Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res. 2000;93:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Hengartner MO. Apoptosis. DNA destroyers. Nature. 2001;412:27, 29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5259] [Cited by in RCA: 5204] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 25. | Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 368] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | 1Petit PX, Susin SA, Zamzami N, Mignotte B, Kroemer G. Mitochondria and programmed cell death: back to the future. FEBS Lett. 1996;396:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 368] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Finkel E. The mitochondrion: is it central to apoptosis. Science. 2001;292:624-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 842] [Cited by in RCA: 818] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 30. | Wakabayashi T. Structural changes of mitochondria related to apoptosis: swelling and megamitochondria formation. Acta Biochim Pol. 1999;46:223-237. [PubMed] |
| 31. | Frey TG, Mannella CA. The internal structure of mitochondria. Trends Biochem Sci. 2000;25:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 557] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 32. | Hirsch T, Marzo I, Kroemer G. Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep. 1997;17:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Zamzami N, Hirsch T, Dallaporta B, Petit PX, Kroemer G. Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. J Bioenerg Biomembr. 1997;29:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 254] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Kroemer G. Mitochondrial control of apoptosis: an overview. Biochem Soc Symp. 1999;66:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Ravagnan L, Marzo I, Costantini P, Susin SA, Zamzami N, Petit PX, Hirsch F, Goulbern M, Poupon MF, Miccoli L. Lonidamine triggers apoptosis via a direct, Bcl-2-inhibited effect on the mitochondrial permeability transition pore. Oncogene. 1999;18:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res. 1999;249:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Anuradha CD, Kanno S, Hirano S. Oxidative damage to mitochondria is a preliminary step to caspase-3 activation in fluoride-induced apoptosis in HL-60 cells. Free Radic Biol Med. 2001;31:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Smaili SS, Hsu YT, Sanders KM, Russell JT, Youle RJ. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ. 2001;8:909-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Skulachev VP. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett. 1998;423:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 377] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Simon DK, Lin MT, Ahn CH, Liu GJ, Gibson GE, Beal MF, Johns DR. Low mutational burden of individual acquired mitochondrial DNA mutations in brain. Genomics. 2001;73:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247-263; discussion 263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Chang SW, Zhang D, Chung HD, Zassenhaus HP. The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer's brain. Biochem Biophys Res Commun. 2000;273:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci USA. 1999;96:4820-4825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 465] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 44. | Lu CY, Lee HC, Fahn HJ, Wei YH. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat Res. 1999;423:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Bhat HK, Hiatt WR, Hoppel CL, Brass EP. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation. 1999;99:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Swerdlow RH, Parks JK, Cassarino DS, Shilling AT, Bennett JP, Harrison MB, Parker WD. Characterization of cybrid cell lines containing mtDNA from Huntington's disease patients. Biochem Biophys Res Commun. 1999;261:701-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998;217:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 517] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 49. | Swerdlow RH, Parks JK, Davis JN, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, Wooten GF. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson's disease family. Ann Neurol. 1998;44:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |