Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.547
Revised: November 7, 2002
Accepted: November 14, 2002
Published online: March 15, 2003
AIM: To investigate the effect of natriuretic peptides on gastric motility in various animals, and the effect of C-type natriuretic peptide (CNP) on spontaneous contraction of gastric smooth muscle in rat, guinea-pig and human in vitro was compared.
METHODS: Spontaneous contraction of gastric smooth muscle was recorded by four channel physiograph.
RESULTS: In the guinea-pig and rat gastric antral circular smooth muscle, CNP markedly decreased the amplitude of spontaneous contraction but it didn’t affect the frequency, however, the contractile activity was completely inhibited by CNP in gastric antral longitudinal smooth muscle. In the human gastric antral circular and longitudinal smooth musle, CNP completely inhibited spontaneous contraction. In the circular smooth muscle of guinea-pig and rat gastric fundus, CNP obviously decreased the amplitude of spontaneous contraction but it didn’t affect the frequency, however, the contractile activity was completely inhibited by CNP in smooth muscle of fundus longitudinal. In the circular and longitudinal smooth muscle of guinea-pig gastric body, CNP at first induced a relaxation and then an increase in amplitude of spontaneous contraction (rebound contraction), but the frequency was not changed. After the circular smooth muscle of gastric body was pretreated with atropine, an M receptor blocker, the rebound contraction was abolished; In circular and longitudinal smooth muscle of rat gastric body, CNP induced a transient and slight relaxation and successively followed by the recovery in amplitude of spontaneous contraction but it also didn’t affect the frequency. After the smooth muscle was pretreated with atropine, the transient and slight relaxation was replaced by long term and complete inhibition; The percentage of CNP-induced inhibition was 76.77% ± 6.21% (fundus), 67.21% ± 5.32% (body) and 58.23% ± 6.21% (antral) in the gastric circular muscle, however, the inhibitory percentage was 100% ± 0.00% (fundus), 68.66% ± 3.55% (body) and 100% ± 0.00% (antrum) in the gastric longitudinal smooth muscle of guinea-pigs; In the rat, the percentage of CNP-induced inhibition was 95.87% ± 4.12% (fundus), 94.91% ± 5.08% (body) and 66.32% ± 7.32% (antrum) in the gastric circular smooth muscle, but in the longitudinal smooth muscle, CNP completely inhibited the spontaneous contraction. Using LY83583, a guanylate cyclase inhibitor, and zaparinast as a phosphoesterase inhibitor to inhibit the generation of cGMP, the effect of CNP on the spontaneous contraction was markedly weakened by LY83583, however, the inhibitory effect was enhanced by zaparinast.
CONCLUSION: (1) CNP can obviously inhibit the spontaneous contraction of gastric antral circular and longitudinal smooth muscle in the rat, guinea-pig and human. The order of inhibitory potency is human > rat > guinea-pig. (2) In the same animals, the inhibitory effect of CNP on spontaneous contraction is the most powerful in fundus and the weakest in antrum, in the same position, the inhibitory effect on the circular smooth muscle is more powerful than that on longitudinal smooth muscle. (3) The inhibitory effect of CNP on spontaneous contraction in the gastric smooth muscle is mediated by a cGMP dependent pathway.
- Citation: Guo HS, Jin Z, Jin ZY, Li ZH, Cui YF, Wang ZY, Xu WX. Comparative study in the effect of C-type natriuretic peptide on gastric motility in various animals. World J Gastroenterol 2003; 9(3): 547-552
- URL: https://www.wjgnet.com/1007-9327/full/v9/i3/547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.547
Since antrial natriuretic peptide (ANP) was isolated from atrium by de Bold et al[1] in 1981, brain natriuretic peptide (BNP), C-type natriurietic peptide (CNP), dendroaspis (DNP), micrurus natriuretic peptide (MNP) and ventricular natriuretic peptide (VNP) were successively found. They distribute in all over the body not only in the heart. Among the natriuretic peptides (NP) family, the most studies were focused on ANP, BNP and CNP. The NP family has function of natriuresis-diuresis, vasorelaxation and is able to lower blood pressure and to keep electrolyte homeostasis and so on. CNP was originally found in the central nervous system of the porcine, but it has recently been found in many other systems. In adult mice, the highest CNP expression was detected in the uterus and ovaries, which exceeded the CNP concentrations of the forebrain and brainstem. In contrast, neonatal mice showed highest CNP-mRNA levels in forebrain and brainstem but with lower levels in the skin, tongue, heart, lung, thymus, skeletal muscle, liver, kidney, stomach, and skull[2]. In the rat, generation and secretion of CNP was demonstrated not only in endothelium but also in vascular smooth muscle cells[3], and natriuretic peptides gene was expressed[4] in human coronary arteries. CNP exhibits many functions besides natriuresis-diuresis; for instance, CNP inhibits the growth of vascular smooth muscle cells in the rat[5] and in has many functions including anti-thrombus and anti-proliferation against vascular smooth muscle cells and myofibroblasts in addition to vasodilation in rabbit[6,7].
The study on the relationship between CNP and gastrointestinal function has recently become the hot spot. In 1991, Komatsu et al[8] demonstrated that CNP also existed in gastrointestinal and three kinds of NPR receptors was found in both mucosa and muscle tissues of the antrum in the rat by Gower et al[9] in 2000. The study on CNP in gastrointestinal tract was mainly focused on physiological functions of absorption, secretion and intestinal motility[10-12]. But few studies reported the regulation of natriuretic peptides on gastric smooth muscles motility. Therefore in the present study, the effect of CNP on spontaneous contraction of gastric smooth muscles in different positions of various animals were investigated.
Wista rats (provided by Experimental Animal Center of Yanbian University medical college) of either sex weighing 250 ± 50 g and EWG/B guinea-pigs (provided by Experimental Animal Center of Jilin University medical college) of either sex weighing 300 ± 50 g were euthanized by lethal dose of intravenous pentobarbital sodium (50 mg/kg). The abdomen of each rat was opened along the midline and stomach was removed and placed in a pre-oxygenated Tyrode’s solution at room temperature. The mucous layer was removed. Strips (about 2.0 × 15.0 mm) of gastric antral circular smooth muscle were prepared by cutting along the vertical direction of the longer axis of the stomach and strips of gastric antral longitudinal smooth muscle were prepared by cutting along the longer axis of the stomach. Muscle strips were placed in a chamber. One end of the strip was fixed on the lid of the chamber through glass claw, the other end was attached to an isometric force transducer (TD-112S, JAPAN) to record the contraction. The chamber (2 mL volume) was constantly perfused with pre-oxygenated Tyrode’s solution at 1 mL/min. Temperature was maintained at 37.0 ± 0.5 °C by a water bath thermostat (WC/09-05, Chongqing, China). The muscle strips were incubated for at least 40 min before experiments were started. The samples of human stomach were offered by Affiliated Hospital of Yanbian University College of Medicine (As the sample of human gastric was limited, only the gastric antral of human was sampled).
Tyrode solution containing (mmol·L-1) 147 NaCl, 4 KCl, 1.05 MgCl2·6H2O, 0.42 CaCl2·2H2O, 1.81 Na2PO4. 2H2O, and 5.5 mM glucose was used. Its pH was adjusted to 7.35 by NaOH. C-type natriuretic peptide and LY83583 was diluted to 10-7 mol·L-1. And, Zaprinast was diluted to 10-6 mol·L-1. All drugs above were purchased from Sigma (USA).
All data was expressed as means ± SD. Statistical significance was evaluated by a t-test. Differences were considered to be significant when P value was less than 0.05.
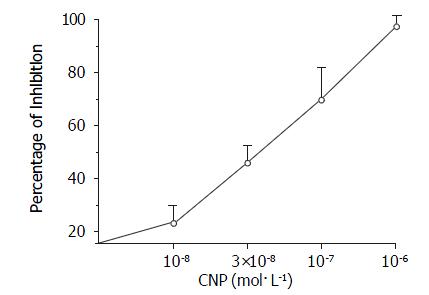
Different concentrations of CNP obviously inhibited spontaneous contraction in a dose-dependent manner and the percentage of inhibition was 22.8% ± 7.2%, 44.9% ± 7.6%, 69.1% ± 12.9% and 98.2% ± 4.7% at the concentrations of 1 × 10-8 mol·L-1, 3 × 10-8 mol·L-1, 1 × 10-7 mol·L-1 and 1 × 10-6 mol·L-1 respectively (Figure 1, n = 7). The middle dose of CNP 10-7 mol·L-1 was used in following all experiments.
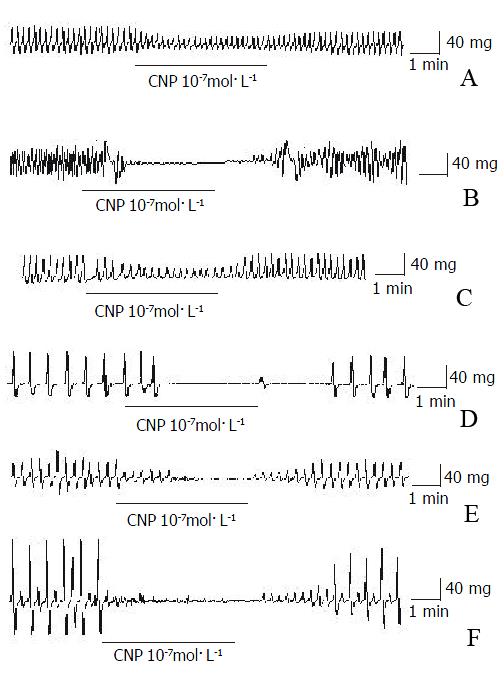
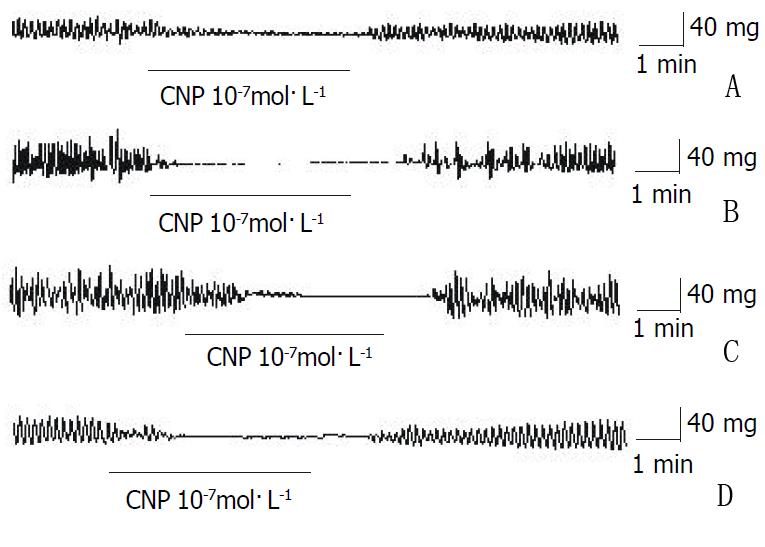
The amplitude of spontaneous contraction in gastric antral circular smooth muscles of guinea-pigs and rats was significantly decreased by CNP and the percentage of inhibition was 58.23% ± 6.21% and 66.32% ± 7.32% respectively, however, the frequency was not influenced CNP (Figure 2A and Figure 2C, Table 1.). The spontaneous contraction of the longitudinal smooth muscle was entirely inhibited by CNP in guinea-pigs and rats (Figure 2B and Figure 2D,Table 1). In the human gastric antrum both circular and longitudinal smooth muscles, the spontaneous contraction was also completely inhibited by CNP (Figure 2E and Figure 2F,Table 1).
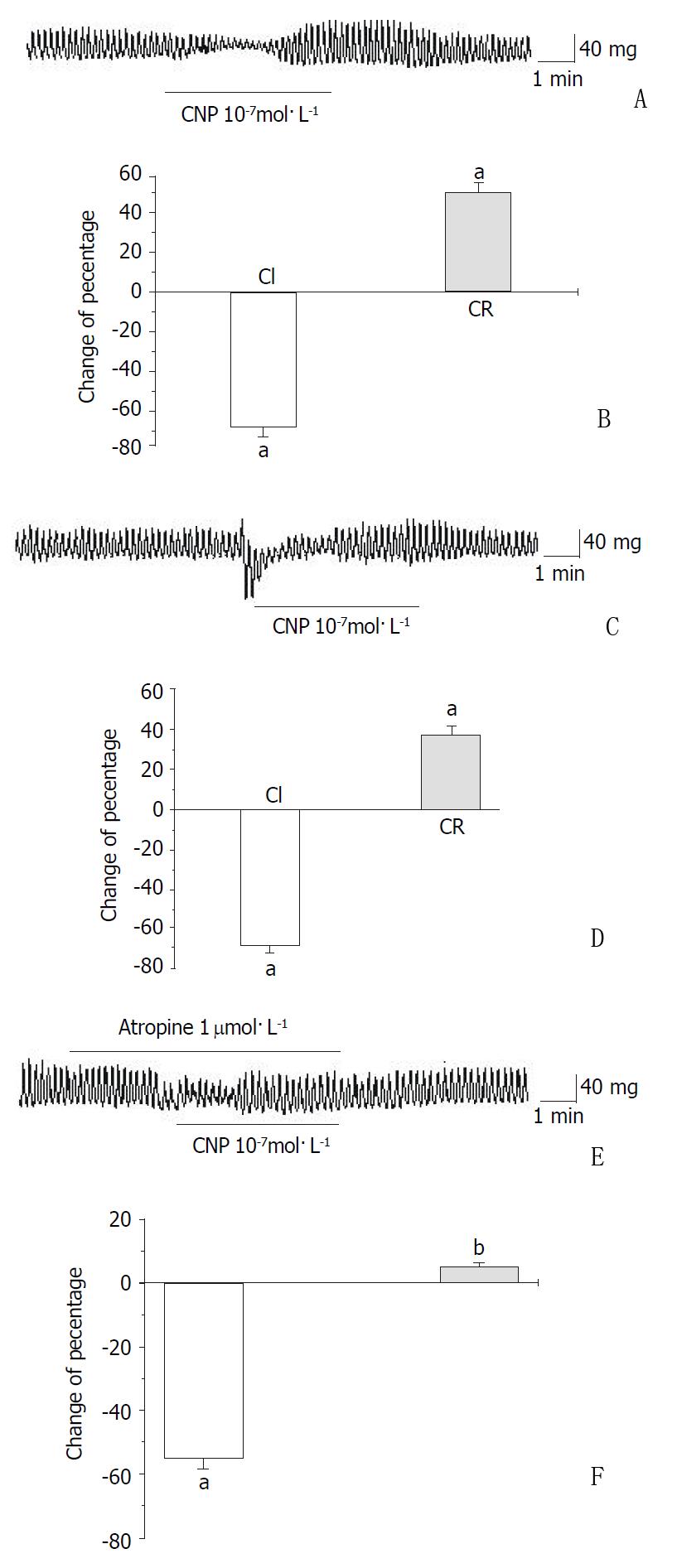
CNP-induced response in the gastric body smooth muscle was different from the response in gastric antral smooth muscle in guinea-pigs and rats. In circular and longitudinal smooth muscles of guinea-pig gastric body, CNP first induced a relaxation and successively an increase in the amplitude of spontaneous contraction (rebound contraction), but the frequency was not changed (Figure 3A and Figure 3C). After the gastric body circular smooth muscle was pretreated with atropine, an M receptor blocker, the rebound contraction was abolished (Figure 3E). In the circular smooth muscle of guinea-pig body, the percentage of inhibition was 67.21% ± 5.32% and the percentage of increase was 50.22% ± 4.67% (Figure 3B). However, in the longitudinal smooth muscle of guinea-pig body, the percentage of inhibiton was 68.66% ± 3.55% and the percentage of increase was 38.91% ± 4.08% (Figure 3D). The frequency was not affected by CNP in the circular and longitudinal smooth muscles of gastric body of the guinea-pig and the pre-and post treat frequency were 6.44 ± 0.64, 6.48 ± 0.67 (circular smooth muscle); 6.53 ± 0.34, 6.55 ± 0.29 (longitudinal smooth muscle) respectively. After treated with atorpine, the percentages of CNP-induced inhibition and increase were 55.33% ± 3.23% and 5.12% ± 1.19% respectively in the gastric body circular smooth muscle of the guinea-pig (Figure 3F). In the circular and longitudinal smooth muscles of rat gastric body, CNP induced a transient and a slight relaxation and it was followed by the recovery in an amplitude of spontaneous contraction, but the frequency was not affected by CNP (Figure 4A and Figure 4C). The percentage of inhibition was 94.91% ± 5.08% in the circular smooth muscle of gastric body and 94.34% ± 5.65% in the g longitudinal smooth muscle of gastric body (Figure 4B and Figure 4D) respectively. After the smooth muscle was treated with atropine, the transient and slight relaxation was replaced by long term and complete inhibition. After treated with atorpine, the inhibitory duration of contractile activity was lengthened from 1.3 ± 0.21 min to 4.88 ± 0.34 min and the rebound contraction disappeared in the gastric body circular smooth muscles of the rat (Figure 4A and Figure 4E).
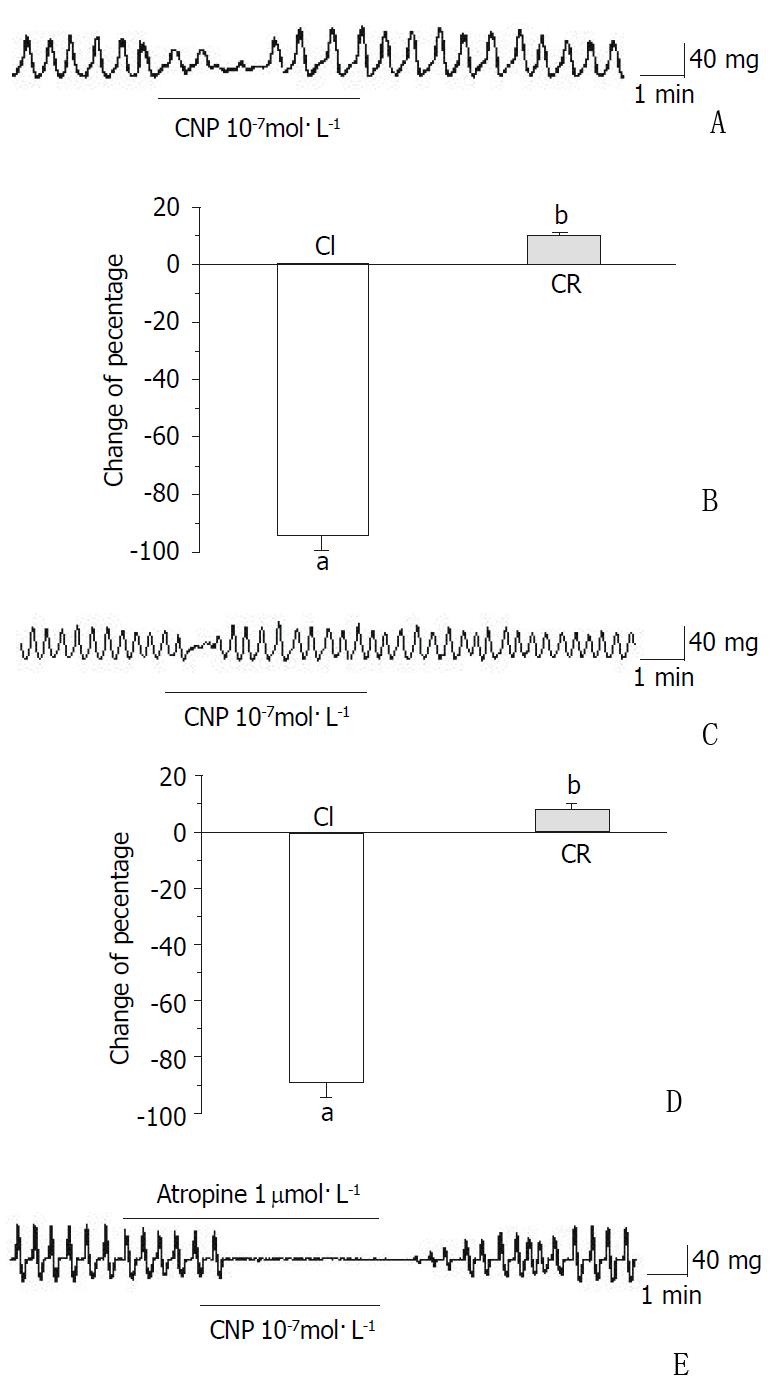
CNP diminished the amplitude of the gastric fundus circular smooth muscles of guinea-pigs and rats, and the percentage of inhibition was 76.77% ± 6.21% and 95.87% ± 4.12%, respectively (Figure 5A and Figure 5B, Table 2). However, the frequency of spontaneous contraction was not affected by CNP in the g circular smooth muscles of gastric fundus of guinea-pigs and rats (Table 2). The spontaneous contraction of gastric fundus longitudinal smooth muscles of guinea-pigs and rats was completely inhibited (Figure 5C and Figure 5D, Table 2).
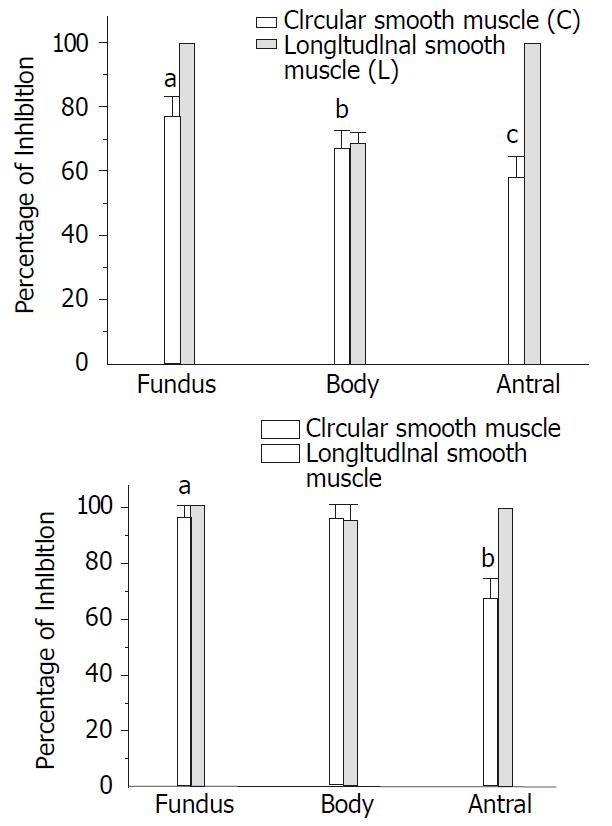
The contractile amplitude was decreased by CNP in the gastric fundus, body and antral circular smooth muscles of the guinea-pig and the percentages of inhibition were 76.77% ± 6.21%, 67.21% ± 5.32% and 58.23% ± 6.21% respectively. However, the percentages of inhibition in longitudinal smooth muscle were 100% ± 0.00%, 68.66% ± 3.55% and 100% ± 0.00% respectively (Figure 6A). The contractile amplitude was decreased by CNP in the gastric fundus, body and antral circular smooth muscles of the rat and the percentages of inhibition were 95.87% ± 4.12%, 94.91% ± 5.08% and 66.32% ± 7.32%, respectively. However, the percentages of inhibition in the longitudinal smooth muscles were 100% ± 0.00%, 94.34% ± 5.65% and 100% ± 0.00% (Figure 6B) respectively.
After the gastric antral circular smooth muscle was treated by LY83583 10-7 mol·L-1 and zaparinast (10-7 mol·L-1) for 15 min to regulate the generation of cGMP, the effect of CNP on gastric motility was observed in the gastric antral circular smooth muscle of the rat. LY83583 markedly diminished the inhibitory effect of CNP on the spontaneous contraction, however, zaparinast enhanced the inhibitory effect of CNP on the spontaneous contraction (Table 3).
In the present study, the effects of CNP on the spontaneous contraction of gastric smooth muscles in the rat, guinea-pig and human were comparatively investigated. The results indicated that CNP markedly inhibited the spontaneous contraction of gastric antral circular smooth muscles in these three kinds of animals and the order of inhibitory potency was human >rat> guinea-pig; CNP inhibited the contractile activity of gastric fundus circular and longitudinal smooth muscles in the rat and guinea-pig and this response was more obvious in the rat than that in guinea-pig; The effects of CNP on gastric body circular and longitudinal smooth muscles had double-phase, in which CNP first induced a relaxation and then an increase in the amplitude of the spontaneous contraction (rebound contraction) in the rat and guinea-pig. The response was more distinct in the guinea-pig than that in the rat and it disappeared after ptretreating with atropine. In the same animal, the CNP-induced inhibition was the most potent in the gastric fundus and the weakest in the gastric antrum, and the inhibition was more obvious in the longitudinal smooth muscle than that in the circular smooth muscle. Using LY83583, a guanylate cyclase inhibitor, and zaparinast as phosphoesterase inhibitor to regulate the generation of cGMP, the effect of CNP on spontaneous contraction was markedly diminished by LY83583. However, the inhibitory effect was enhanced by zaparinast. It was similar to the result observed in intestinal smooth muscle by Murthy et al[13].
Since CNP was isolated from the central nerve system[14], previous studies indicated that as a neurotransmitter, CNP exerted functions in the central nervous system[15] and participated in the regulation of physiological functions in many other systems. CNP was successively found in the cardiovascular system[16,17], respiratory system[18], urinary system[19], reproductive system [2] and sense system[20]. Since Komatsu et al[8] found CNP in the gastrointestinal tracts of humans and rats, there were more and more studies on the relationship between CNP and the gastrointestinal tract. But the study on physiological functions of CNP in gastrointestinal tract was mainly focused on absorption, secretion and intestinal motility[10-12]. Serial studies on natriuretic peptides regulating gastric smooth muscles motility were not reported. In this study, the effect of CNP on gastric motility in various animals, in different positions and in different smooth muscles of the same position of the same animal was comparatively investigated.
In various animals, the effects of CNP on the gastric motility of antrum and fundus smooth muscles presented species diversity. The order of inhibitory potency in gastric antrum was human > rat > guinea-pig, and in gastric fundus, the order was rat > guinea-pig. Analyzing this from the animal characters, It was presumed that either the distribution of the CNP receptor in gastric smooth muscle of three kinds of animals was different or the sensitivity of CNP to CNP receptor in different animal was diverse.
In different positions of the same animal, the effect of CNP on gastric motility exhibited positional diversity. CNP-induced inhibition was the most potent in gastric fundus and the weakest in gastric antrum. The responses may be related to the structure and function of the stomach. It was thought that the muscle layer was the thickest in the gastric antrum and the thinnest in the gastric fundus, and the gastric antrum played an important role in gastric emptying while gastric fundus was related to receptive relaxation. It indicated that the contractile function of the gastric antrum was more potent than the function of gastric fundus. In the same postion of the same animal, the inhibition was more obvious in longitudinal smooth muscles than that in circular smooth muscles. The reason was probably that the distribution of CNP receptors showed diversity.
It is well known that NP and NO system are all cGMP-generation system. They play a very impotant role in regulation of multiple physiological functions. To investigate the mechanism of CNP-induced inhibition, LY83583, a kind of inh ib ito r o f gu any late cy clase, an d zap arin ast, a phosphoesterase inhibitor were used for observing the effect of CNP on gastric motility in gastric antral circular smooth muscle of the rats. LY83583 markedly diminished the inhibitory effect of CNP on the spontaneous contraction, however, zaparinast enhanced the inhibitory effect of CNP on the spontaneous contraction. It indicated that CNP-induced inhibition on the spontaneous contraction in the gastric smooth muscle via the cGMP dependent pathway. Previous studies also surported the theory, for example, CNP dilated the vascular smooth muscle[21] and relaxed the bronchus smooth muscle[22] via cGMP dependent pathway.
In this present study, the CNP-induced response on spontaneous contraction of the gastric body showed double-phase, in which CNP first induced a relaxation and then inducing rebound contractions in rats and guinea-pigs. The response was more distinct in guinea-pigs than that in rat and it disappeared after pretreated with atropine. The results indicated that cholinergic M receptor participated in this CNP-induced rebound contraction of gastric body smooth muscle in guinea-pigs and rats. Previous studies also indicated that vagus nerve regulated many functions of NP, for example, ANP promoted gastric acid secretion[23], ANP, BNP and CNP could all induce bradycardia[24] and BNP and CNP could enhance vagus-induced response of slowing heart rate[25]. Then, it was suggested that CNP may facilitate the cholinergic nerve activity in gastric smooth muscles.
CNP may participate in the regulation of gastric motility as gastrointestinal hormone or nurotransmitter. CNP-induced inhibition has species diversity and position diversity in gastric smooth muscles. CNP-induced inhibition on gastric motility may be related to the increase of intracellular cGMP.
Edited by Xu XQ
| 1. | de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Reprinted from Life Sci. 28: 89-94, 1981. J Am Soc Nephrol. 2001;12:403-409; discussion 403-409; 403-409;. [PubMed] |
| 2. | Stepan H, Leitner E, Bader M, Walther T. Organ-specific mRNA distribution of C-type natriuretic peptide in neonatal and adult mice. Regul Pept. 2000;95:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Woodard GE, Rosado JA, Brown J. Expression and control of C-type natriuretic peptide in rat vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:R156-R165. [PubMed] |
| 4. | Casco VH, Veinot JP, Kuroski de Bold ML, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem. 2002;50:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Furuya M, Yoshida M, Hayashi Y, Ohnuma N, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1991;177:927-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Wang X, Xue L, Tong L. [Influence of vasoactive peptides on homocysteine-induced proliferation of cultured rabbit vascular smooth muscle cell]. Zhonghua Yixue Zazhi. 1999;79:411-413. [PubMed] |
| 7. | Ohno N, Itoh H, Ikeda T, Ueyama K, Yamahara K, Doi K, Yamashita J, Inoue M, Masatsugu K, Sawada N. Accelerated reendothelialization with suppressed thrombogenic property and neointimal hyperplasia of rabbit jugular vein grafts by adenovirus-mediated gene transfer of C-type natriuretic peptide. Circulation. 2002;105:1623-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Komatsu Y, Nakao K, Suga S, Ogawa Y, Mukoyama M, Arai H, Shirakami G, Hosoda K, Nakagawa O, Hama N. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology. 1991;129:1104-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 228] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Gower WR, Salhab KF, Foulis WL, Pillai N, Bundy JR, Vesely DL, Fabri PJ, Dietz JR. Regulation of atrial natriuretic peptide gene expression in gastric antrum by fasting. Am J Physiol Regul Integr Comp Physiol. 2000;278:R770-R780. [PubMed] |
| 10. | Vuolteenaho O, Arjamaa O, Vakkuri O, Maksniemi T, Nikkilä L, Kangas J, Puurunen J, Ruskoaho H, Leppäluoto J. Atrial natriuretic peptide (ANP) in rat gastrointestinal tract. FEBS Lett. 1988;233:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Brockway PD, Hardin JA, Gall DG. Intestinal secretory response to atrial natriuretic peptide during postnatal development in the rabbit. Biol Neonate. 1996;69:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Akiho H, Chijiiwa Y, Okabe H, Harada N, Nawata H. Interaction between atrial natriuretic peptide and vasoactive intestinal peptide in guinea pig cecal smooth muscle. Gastroenterology. 1995;109:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Murthy KS, Teng B, Jin J, Makhlouf GM. G protein-dependent activation of smooth muscle eNOS via natriuretic peptide clearance receptor. Am J Physiol. 1998;275:C1409-C1416. [PubMed] |
| 14. | Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 728] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Minerds KL, Donald JA. Natriuretic peptide receptors in the central vasculature of the toad, Bufo marinus. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Doyle DD, Upshaw-Earley J, Bell EL, Palfrey HC. Natriuretic peptide receptor-B in adult rat ventricle is predominantly confined to the nonmyocyte population. Am J Physiol Heart Circ Physiol. 2002;282:H2117-H2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Wright RS, Wei CM, Kim CH, Kinoshita M, Matsuda Y, Aarhus LL, Burnett JC, Miller WL. C-type natriuretic peptide-mediated coronary vasodilation: role of the coronary nitric oxide and particulate guanylate cyclase systems. J Am Coll Cardiol. 1996;28:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Nakanishi K, Tajima F, Itoh H, Nakata Y, Hama N, Nakagawa O, Nakao K, Kawai T, Torikata C, Suga T. Expression of C-type natriuretic peptide during development of rat lung. Am J Physiol. 1999;277:L996-L1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Meier SK, Toop T, Donald JA. Distribution and characterization of natriuretic peptide receptors in the kidney of the toad, Bufo marinus. Gen Comp Endocrinol. 1999;115:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Suzuki M, Kitanishi T, Kitano H, Yazawa Y, Kitajima K, Takeda T, Tokunaga Y, Maeda T, Kimura H, Tooyama I. C-type natriuretic peptide-like immunoreactivity in the rat inner ear. Hear Res. 2000;139:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Wennberg PW, Miller VM, Rabelink T, Burnett JC. Further attenuation of endothelium-dependent relaxation imparted by natriuretic peptide receptor antagonism. Am J Physiol. 1999;277:H1618-H1621. [PubMed] |
| 22. | Borges A, de Villarroel SS, Winand NJ, de Bécemberg IL, Alfonzo MJ, de Alfonzo RG. Molecular and biochemical characterization of a CNP-sensitive guanylyl cyclase in bovine tracheal smooth muscle. Am J Respir Cell Mol Biol. 2001;25:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Puurunen J, Ruskoaho H. Vagal-dependent stimulation of gastric acid secretion by intracerebroventricularly administered atrial natriuretic peptide in anaesthetized rats. Eur J Pharmacol. 1987;141:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Thomas CJ, May CN, Sharma AD, Woods RL. ANP, BNP, and CNP enhance bradycardic responses to cardiopulmonary chemoreceptor activation in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2001;280:R282-R288. [PubMed] |
| 25. | Herring N, Zaman JA, Paterson DJ. Natriuretic peptides like NO facilitate cardiac vagal neurotransmission and bradycardia via a cGMP pathway. Am J Physiol Heart Circ Physiol. 2001;281:H2318-H2327. [PubMed] |