Published online Mar 15, 2003. doi: 10.3748/wjg.v9.i3.479
Revised: August 20, 2002
Accepted: October 12, 2002
Published online: March 15, 2003
AIM: To develop atumor vaccine by fusion of H22 hepatocarcinoma cells and DC, and to study its protective and therapeutical effect against H22 cell.
METHODS: H22-DC vaccine was produced by PEG fusion of H22 and DC induced by cytokine released from splenic mononuclear cells, sorted by CD11c magnetic microbead marker. It was injected through the tail vein of the mice and the H22-DC oncogenesis was detected in the liver, spleen and lung. In order to study the therapeutical and protective effect of H22-DC against tumor H22, two groups were divided: immune group and therapeutic group. Immune group was further divided into P, D, HD and H subgroups, immunized by PBS, DC, H22-DC and inactivated H22, respectively, and attacked by H22 cell. The tumor size, tumor weight, mice survival time and tumor latent period were recorded and statistically analyzed; Therapeutical group was divided into three subgroups of P, D and HD, and attacked by H22, then treated with PBS, DC, and H22-DC, respectively. Pathology and flow cytometry were also applied to study the mechanism how the H22-DC vaccine attacked on the H22 cell.
RESULTS: 1. No oncogenesis was found in spleen, lung and liver after H22-DC injection. 2. Hybrid vaccine immunized mice had strongest CTL activity. 3. In the immune group, latent period was longer in HD subgroup than that in P, H and D subgroup; and tumor size and weight were smaller in HD subgroup than that in P, H and D subgroup. 4. In therapeutic group, tumor size was smaller in HD subgroup than that in P, D subgroup.
CONCLUSION: 1. H22-DC tumor vaccine is safe without oncogenesis in vivo. 2. Hybrid vaccine can stimulate potent specific CTL activity against H22. 3. H22-DC vaccine has distinctive prophylatic effect on tumor H22 and can inhibit the tumor growth.
-
Citation: Zhang JK, Li J, Zhang J, Chen HB, Chen SB. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma
in vivo . World J Gastroenterol 2003; 9(3): 479-484 - URL: https://www.wjgnet.com/1007-9327/full/v9/i3/479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i3.479
Tumor cells have low antigenicity and high antigen modulatory ability, by which tumor associated antigen or tumor specific antigen can’t be efficiently presented to T cells and tumor specific killing T cell can’t be efficiently activated[1-5]. Furthermore, as one of antigen presenting cells (APC), tumor cells are anergy to T cells because of their deficiency of costimulator. Dendritic cells (DC), as a potent professional APC, not only express MHC molecules and costimulators to present tumor antigen and activate tumor specific T cells, but also can directly activate NK cell to kill MHC molecule negative tumor cell in nonspecific immunity. So much attention was paid to DC for its potential application in antitumor immunity[6-10]. Hepatocarcinoma is a high malignant tumor, so it is an important assignment to find a suitable way to kill the hepatocarcinoma. Our previous work showed that hybrid vaccine could kill tumor cell in vitro[11-16]. In this report, we tried to find a way to apply the DC in the prevention and therapy of hepatocarcinoma.
Tumor cell line H22 cell line, constructed by Dalian medical university from the mouse ascite, was heterogeneous cells with higher declination to spread by lymph vessel.
Animal BALB/c mice were bought from laboratory animal center of antibiotic industrial institute in Sichuan china. All mice were male, specific pathogen free animals, with age of 6-8 weeks and weight of 15-20 g.
Main reagents Metrizamide was product of Amresco Company. Mini MACS cell sorter and CD11c MicroBeads marked antibody were products of Miltenyi Biotec GmbH Company. rmGM-CSF (granulocyte-macrophage colony-stimuting factor) was a product of R & D company. Polyethylene glycol (PEG) was purchased from Sigma Chemical Company. MTT solution was a product of Amresco Company.
Culture of tumor cells H22 cell was incubated in double-layer agarose culture medium (upper layer 0.3%, base layer 0.6%) with concentration of 5 × 105/L and cultured for 10 days. Single colony was selected and transferred to culture flask for expanding culture. Cells of the logarithmic stage were collected and conserved in frozen conditions for later use. 0.1 mL 1 × 107 H22 cells were incubated subcutaneously at the right armpit, carcinogenesis in vivo was observed.
Isolation of DC from spleen Spleen of mice was grinded it into cell suspension, they were lysed by NH4Cl, centrifugated by metrizamide gradient solution. Cells in the interface were collected, and cultured routinely by complete culture medium RPMI 1640 at 100% humidity, 37 °C, 50 mL·L-1 CO2 for 3 hs, then the suspended cells were removed and continued to be cultured for another 16 hs, the non-adherent cells were collected, and adjusted to the concentration of 1 × 109/L, then cultured in the complete medium with 200 mL·L-1 NCS and rmGM-CSF (500 ng/L) for 5-7days[17].
Fusion of DCs and H22 DCs and H22 were washed by PBS for 3 times, then the mixture of these two kinds of cells (DCs: H22 = 1:1) were centrifugated to remove the upper liquid gently and completely, the cell droplet was loosen by shaking, 500 mL·L-1 PEG was added in the droplet in 37 °C water bath to fuse for 1 min, and simultaneously the tube was shook gently. D-hanks solution was added to terminate the fusion process for 5 min in 37 °C water bath, the upper liquid was removed by centrifugation, and the cell droplet was collected. the cell availability and number of fusion cells was detected by trypan blue staining.
Screening and determination of hybrid cell The fusion cells were marked by CD11c antibody, and sorted by Mini MACS to remove the CD11c_ cells and the CD11c+ cells were collected and they were cultured in RPMI complete medium with rmGM-CSF (500 ng/L) and 200 mL·L-1 NCS for 2-3 weeks[18].
Carcinogenic effect of hybrid vacciney in vivo Mice were divided into five groups of HD1group, HD2 group, D group, H group and P group. 0.1 mL hybrid vaccine was injected into tail veil of mice in the HD1group, mice in the other group were injected at right armpit subcutaneously with 0.1 mL 1-2 × 107/mL vaccine, H22 cells+DC, H22 cells and PBS, respectively. And, 14 days later, tissues of the injection site, spleen, liver and lung were isolated, the tumor weight was compared.
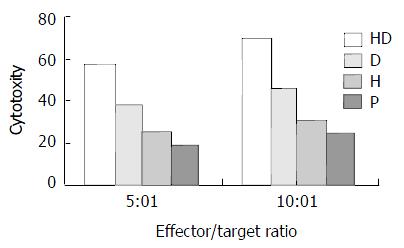
Analysis of the CTL activity 20 mice were classified to four groups of HD group, D group, H group and P group, each group had 5 mice. Mice were injected with 0.1 mL hybrid vaccine, DC, heat inactivated H22 and PBS at the concentration of 1 × 1010-2 × 1010/L, twice every three days. All the mice were killed at 10 days after the last immunization and the spleen lymphocytes were separated and cultured under condition of complete medium with IL-2 (1 × 105u/L) and 100 mL·L-1 FCS at saturated humidity, 37 °C, 50 mL·L-1 CO2 to induce the CTL. CTL and H22 was mixed at 5:1 and 10:1 effector/target rate, respectively. In addition, there were a CTL control group, a H22 control group and a culture medium control group. All specimens had 3 parallel wells on a 96-well culture plate. All were cultured under conditions of saturated humidity 37 °C, 50 mL·L-1 CO2 for 48 hs. MTT method was used to detect the CTL cytotoxity against H22. Chief process was as below: adding 5 g/L MTT solution 20 μL in each well for 4 h before the detection, then it was centrifugated to remove the free MTT; 150 μL DMSO was added to the cell droplet for 10 min to solve the MTT. Bio-Rad 350-uv automatic enzyme linker detector is used to detect the OD value at 570 wavelength[19]. All were repeated for 4 times.
Protective effect of hybrid vaccine against H22 40 BALB/c mice were randomly divided into HD, D, H and P subgroups, ten mice in each subgroup, immunized by 0.1 mL hybrid vaccine, DC, heat inactivated H22 and PBS, respectively, at concentration of 1 × 109/L, two times every three days by tail veil. Three days after the last immunization, all mice were injected by 1 × 106 H22 at the right armpit subcutaneously. The mice weight, tumor formation, tumor size and day of mice death were recorded. 5 mice were randomly chose and killed on day 14, tumor tissue, lung tissue, liver tissue and lymph node of neck and armpit were sampled for routine paraffin embedded slice and HE staining. Other mice were kept observation for 50 days.
Therapeutic effect of hybrid vaccine against H22 30 BALB/ c mice were randomly divided into HD, D and P subgroups, ten mice in each subgroup. All mice was injected with H22 tumor cell 1 × 106 subcutaneously at right armpit. 3 days later, tumor was formed in all mice which demonstrated the successful construction of tumor model. Mice in different groups were treated with 0.1 mL hybrid vaccine, DC, and PBS, respectively, at the concentration of 1 × 109/L, two times every three days, by the tail veil. The tumor weight and size were recorded each day. 5 mice were randomly chose and killed on day 14; pathological changes were observed in tumor tissue. The other mice were kept on feeding for 50 days.
Data were analyzed by the method of ANOVA (SPSS 10.0 software).
H22 grew in vitro in a suspended manner, single or integrated, its double proliferation time was 24 hs. H22 cell can grow to form colony composed of hundreds of cells in 5-6 days, thousands cells in ten days on agarose cultured medium.
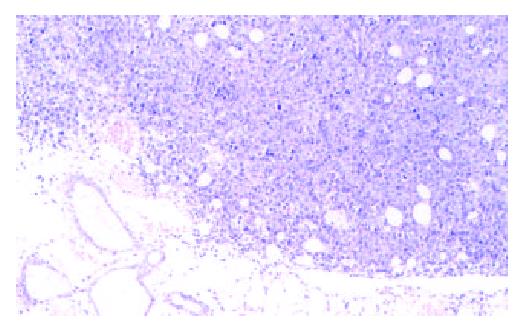
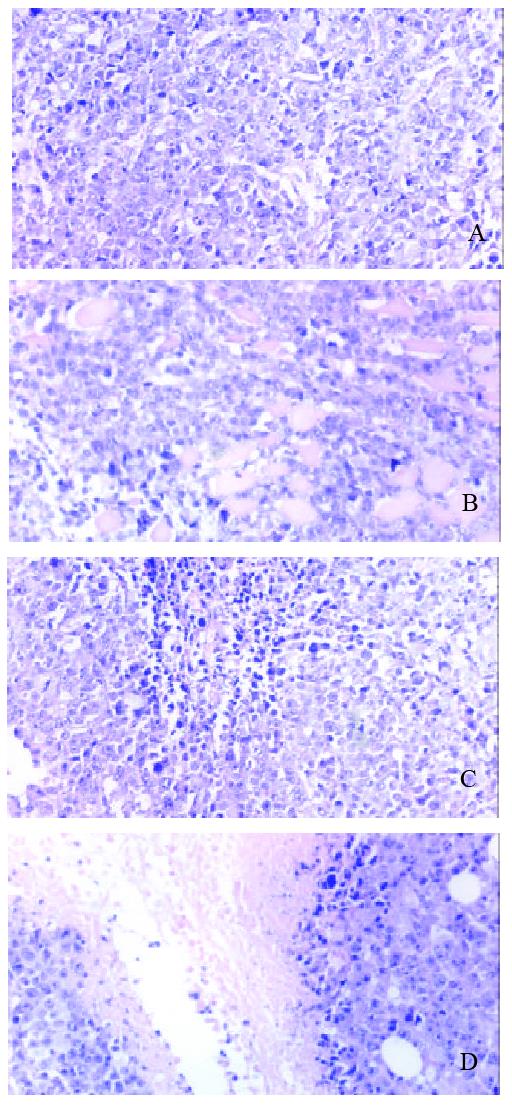
Incubation of 1 × 106 H22 at right armpit subcutaneously in vivo can successfully induce tumor in all mice. Tumor grew aggressively to invade the surrounding tissue, tumor appeared necrosis when tissue bulk were big enough, even gangrenous impairment appeared on skin when tissue diameter was beyond 30 mm. Tumor tissue had integrated cell with few intercellular materials, had no funicular structure of normal liver. Tumor cells had distinctive cellular pleomorphism, nuclear pleomorphism, nuclear hyperchromatism and increased nuclear: cytoplasmic ratio (Figure 1).
DC was round and irregular with sharp and long, or blunt cell extension. Induced by rmGM-CSF for 5-7days, DC grew into a colony; outer cell of it had much extension. Sorted by Microbead marked CD11c, most of them were CD11c+.
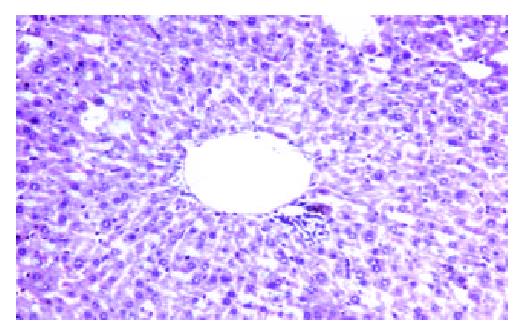
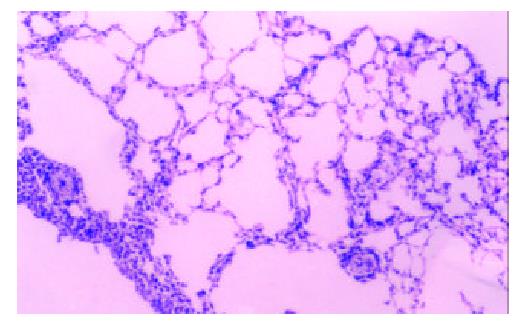
No carcinogenesis appeared after hybrid vaccine was injected subcutaneously for 60 days, while H22 was injected, tumor appeared in 100% mice at site of injection. There was significant in tumor weight between the HD and H22 subgroup (P < 0.01). 1 × 106 hybrid vaccine was injected by tail veil, the mice’s spleen, lung and liver were sampled after 14 days, three slices were sectioned for each organ, no carcinogenesis appeared (Figure 2 and Figure 3).
CTL activity of mice in HD subgroup was significantly higher than that of D, H and P subgroup (Figure 4).
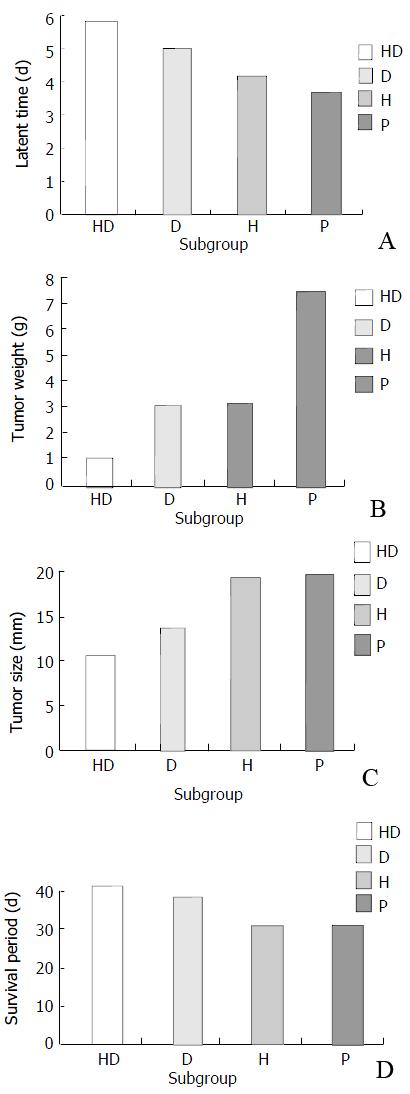
Observation of carcinogenesis Mice of different subgroups in protective group were injected with H22 cells, the tumor latent time ranked as P < H < D < HD subgroup (P < 0.05). This demonstrated that the HD subgroup had delayed tumor latent time (Figure 5A); tumor size and tumor weight ranked as HD < D < H < P subgroup (P < 0.05) (Figure 5B and C), which showed that mice in the HD subgroup had the lowest tumor size and tumor weight, and the D subgroup had secondly lowest tumor size and tumor weight. Survival time among different subgroups had no significant difference (P > 0.05) (Figure 5D).
Pathology of different subgroups Macroscopic structure showed the tumor tissue was hard and adherenced to the surrounding tissue. Cutting face of tumor tissue was gray and diffused dot-like brown. Microscopic structure showed that tumor had dot like or sheet like necrosis (Figure 6).
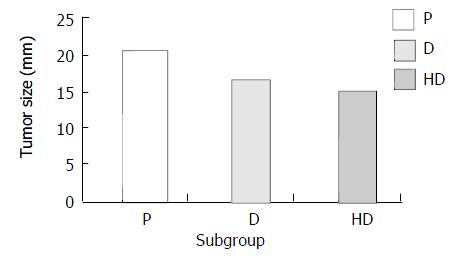
Inhibitory effect of hybrid vaccine in carcinogenesis Treated with hybrid vaccine, DC and PBS, tumor size in different subgroups ranked as HD < D < P (P < 0.05) (Figure 7). Survival time and tumor weight had no significant difference among the different subgroups in therapeutic group.
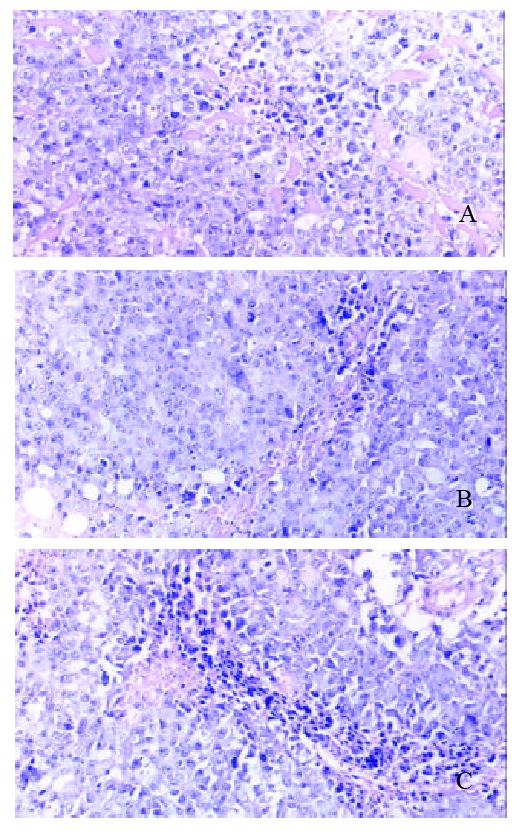
Pathology of different subgroups Macroscopic structure showed the tumor tissue was hard and can’t separate with surrounding tissue easily. Cutting face of tumor tissue is gray and diffuse dot-like brown. Microscopic structure showed tumor has dot like or sheet like necrosis (Figure 8).
H22 ascite hepatocarcinoma has strong invasion and spreading declination by lymph vessel. In this report, H22 cells had 100% carcinogenesis effect on BALB/c mice, which was consistent with other report[19]. H22 cells grow quickly in vitro, which ccan be easily used to construct the tumor animal model, and express low level of MHC molecule. So H22 cells are suitable for DC antitumor research.
In this report, DC was separated through centrifugation by metrizamide; the maturation of DC was induced by cytokine of rmGM-CSF; DC and H22 were fused through PEG, they were marked by CD11c, and sorted by Mini MACS. By above process, the hybrid vaccine was successfully constructed[15,17,19,20]. The methods were simple and easily operated. Hybrid Vaccine, injected subcutaneously, has no tumor formation at local tissue, and injected by tail veil, it also has no carcinogenesis in the liver, lung and spleen, so it loses the carcinogenic effect of H22 cells, and is safe to develop tumor vaccine in vivo.
In the protective group, different subgroups had different tumor latent time, and different interval between the H22 incubation and tumor formation. The HD subgroup had the longest tumor latent time; the D subgroup had the second longest tumor latent time. The results showed that the hybrid vaccine and DC could delay the tumor carcinogenesis. The HD subgroup had the lowest tumor size and tumor weight, the D subgroup had the second lowest tumor size and tumor weight. The results showed that hybrid vaccine had the strongest anti-tumor effect. Hybrid vaccine could express some DC characteristics such as membrane molecule MHC-I, II molecule and costimulator, secrete some cell factors which can stimulate the T cell expansion, capture and present endogenous tumor associated antigens derived form parents H22 cells, so it can efficiently stimulate the immune response[21-25]. There were similar results in other research reports on hybrid vaccines of DC with MC38, NS1, B16 melanoma, RMA-s lymphpma and renal carcinoma[26-35].
Mice immunized by hybrid vaccine could induce the tumor specific memory T cells, which could quickly be activated and expanded when they contact with tumor antigen again. When CD40 which was expressed by memory T cell integrated with CD40L of DC, it could stimulate DC to secrete high level of IL-12, and to enhance the expression of ICAM-1, CD80 and CD86. The activated DC could stimulate the proliferation of T cells and secretion of IFN-γ of T cells[36-39]. It was reported that IFN-γ gene modified H22 cells could enhance the antigenicity and the expresseion of MHC-II molecule from 10% to 19%; TNF gene modified H22 cell also could enhance the MHC-I molecule expression in different clones with different transfection methods which was enhanced to 28.39% and 35.78% in two clones which had the highest expression of MHC-I ; GM-CSF gene modified H22 had lower oncogenicity in vivo, the possible mechanism attributed to the enhanced MHC expression[40-44].
Hybrid vaccine also takes part in nonspecific immunity; it can directly activate NK cells to kill MHC-tumor cells. Some reports demonstrated DC could produce large number of INF-γ, which can inhibit the virus replication and stimulate the NK and macrophages[45-47]. In this report, the hybrid vaccine antitumor function perhaps attributed to both adaptive and inborn immunity.
In the therapeutic group, tumor size in the HD subgroup was significantly lower than that in other subgroups after treated for 14 days, which showed that H22-DC could inhibit the tumor growth. Some reported the similar effect. For example, after breast cancer cells hybridised with CD14+ derived DC, 3H incorporation assay showed that hybrid cell can stimulate the proliferation T cells, while only tumor cell, DC and mixture of DC and tumor cell have no such effect[48-55].
From above, it is concluded that hybrid vaccine acquired the function of parents cell such as antigen presenting function of DC and T stimulating ability and capture the tumor derived antigen. Hybrid vaccine has the protective and therapeutic effect against H22. This research can provide some evidence for the clinical application of tumor vaccine.
Edited by Xu XQ
| 1. | Cohen PA, Peng L, Kjaergaard J, Plautz GE, Finke JH, Koski GK, Czerniecki BJ, Shu S. T-cell adoptive therapy of tumors: mechanisms of improved therapeutic performance. Crit Rev Immunol. 2001;21:215-248. [PubMed] |
| 2. | Nawrocki S, Wysocki PJ, Mackiewicz A. Genetically modified tumour vaccines: an obstacle race to break host tolerance to cancer. Expert Opin Biol Ther. 2001;1:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Azuma I, Seya T. Development of immunoadjuvants for immunotherapy of cancer. Int Immunopharmacol. 2001;1:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 513] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Renner C, Kubuschok B, Trümper L, Pfreundschuh M. Clinical approaches to vaccination in oncology. Ann Hematol. 2001;80:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Nishioka Y, Hua W, Nishimura N, Sone S. Genetic modification of dendritic cells and its application for cancer immunotherapy. J Med Invest. 2002;49:7-17. [PubMed] |
| 7. | Gunzer M, Grabbe S. Dendritic cells in cancer immunotherapy. Crit Rev Immunol. 2001;21:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Brossart P, Wirths S, Brugger W, Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001;29:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Meidenbauer N, Andreesen R, Mackensen A. Dendritic cells for specific cancer immunotherapy. Biol Chem. 2001;382:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Bhardwaj N. Processing and presentation of antigens by dendritic cells: implications for vaccines. Trends Mol Med. 2001;7:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Sun JL, Zhang JK, Chen HB, Chen JD, Qiu YQ. Promoting effects of dendritic cells on LPAK cells killing human hepatoma cells. Zhongguo Zhongliu Lingchuang yu Kangfu. 1998;5:16-18. |
| 12. | Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hapatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673-675. |
| 13. | Zhang JK, Sun JL, Chen HB, Zeng Y, Qu YJ. Influence of granulocyte macrophage colony stimulating factor and tumor necrosis factor on anti-hepatoma activities of human dendritic cells. World J Gastroenterol. 2000;6:718-720. [PubMed] |
| 14. | Sun J, Zhang J, Chen J, Chen H, Chew Y, Chen J. In vitro study on the morphology of human blood dendritic cells and LPAK cells inducing apoptosis of the hepatoma cell line. Chin Med J (Engl). 2001;114:600-605. [PubMed] |
| 15. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |
| 16. | Zhang JK, Li J, Chen HB, Sun JL, Qu YJ, Lu JJ. Antitumor activities of human dendritic cells derived from peripheral and cord blood. World J Gastroenterol. 2002;8:87-90. [PubMed] |
| 17. | Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendritic cells against human hepatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:191-193. |
| 18. | Sun JL, Zhang JK, Chen HB, Zhou YQ. Morphology of cultured human peripheral blood dendritic cells and their antitumor activity. Zhongguo Zuzhihuaxue Yu Xibaohuaxue Zazhi. 1999;8:28-31. |
| 19. | Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hapatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673-675. |
| 20. | Zhang JK, Sun JL, Chen HB, Zhou YQ. Ultrastructural compari-son of apoptosis of human hepatoma cells and lak cells. Huaren Xiaohua Zazhi. 1998;6:877-879. |
| 21. | Hart I, Colaco C. Immunotherapy. Fusion induces tumour rejection. Nature. 1997;388:626-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | van Schooten WC, Strang G, Palathumpat V. Biological properties of dendritic cells: implications to their use in the treatment of cancer. Mol Med Today. 1997;3:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Kolb HJ, Holler E. Adoptive immunotherapy with donor lymphocyte transfusions. Curr Opin Oncol. 1997;9:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Garcia-Prats MD, DeLeo AB, Lotze MT. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells. 1997;15:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Slingluff CL. Tumor antigens and tumor vaccines: peptides as immunogens. Semin Surg Oncol. 1996;12:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, Dalgleish AG. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta. 2000;1500:265-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci USA. 2000;97:2715-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Cao X, Zhang W, Wang J, Zhang M, Huang X, Hamada H, Chen W. Therapy of established tumour with a hybrid cellular vaccine generated by using granulocyte macrophage colony stimulat-ing factor genetically modified dendritic cells. Immunology. 1999;97:616-625. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516-5524. [PubMed] |
| 30. | Celluzzi CM, Falo LD. Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J Immunol. 1998;160:3081-3085. [PubMed] |
| 31. | Lespagnard L, Mettens P, Verheyden AM, Tasiaux N, Thielemans K, van Meirvenne S, Geldhof A, De Baetselier P, Urbain J, Leo O. Dendritic cells fused with mastocytoma cells elicit therapeutic antitumor immunity. Int J Cancer. 1998;76:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 207] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Gong J, Apostolopoulos V, Chen D, Chen H, Koido S, Gendler SJ, McKenzie IF, Kufe D. Selection and characterization of MUC1-specific CD8+ T cells from MUC1 transgenic mice immunized with dendritic-carcinoma fusion cells. Immunology. 2000;101:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Gong J, Nikrui N, Chen D, Koido S, Wu Z, Tanaka Y, Cannistra S, Avigan D, Kufe D. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol. 2000;165:1705-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Holmes LM, Li J, Sticca RP, Wagner TE, Wei Y. A rapid, novel strategy to induce tumor cell-specific cytotoxic T lymphocyte responses using instant dentritomas. J Immunother. 2001;24:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Akasaki Y, Kikuchi T, Homma S, Abe T, Kofe D, Ohno T. Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J Immunother. 2001;24:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Tüting T, Storkus WJ, Lotze MT. Gene-based strategies for the immunotherapy of cancer. J Mol Med (Berl). 1997;75:478-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 425] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 39. | Gluckman JC, Canque B, Chapuis F, Rosenzwajg M. In vitro generation of human dendritic cells and cell therapy. Cytokines Cell Mol Ther. 1997;3:187-196. [PubMed] |
| 40. | Asher AL, Mulé JJ, Kasid A, Restifo NP, Salo JC, Reichert CM, Jaffe G, Fendly B, Kriegler M, Rosenberg SA. Murine tumor cells transduced with the gene for tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991;146:3227-3234. [PubMed] |
| 41. | Porgador A, Tzehoval E, Vadai E, Feldman M, Eisenbach L. Immunotherapy via gene therapy: comparison of the effects of tumor cells transduced with the interleukin-2, interleukin-6, or interferon-gamma genes. J Immunother Emphasis Tumor Immunol. 1993;14:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Zhang LH, Pan JP, Yao HP, Sun WJ, Xia DJ, Wang QQ, He L, Wang J, Cao X. Intrasplenic transplantation of IL-18 gene-modified hepatocytes: an effective approach to reverse hepatic fibrosis in schistosomiasis through induction of dominant Th1 response. Gene Ther. 2001;8:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Lu Y, Yamauchi N, Koshita Y, Fujiwara H, Sato Y, Fujii S, Takahashi M, Sato T, Kato J, Yamagishi H. Administration of subtumor regression dosage of TNF-alpha to mice with pre-existing parental tumors augments the vaccination effect of TNF gene-modified tumor through the induction of MHC class I molecule. Gene Ther. 2001;8:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | van Slooten ML, Storm G, Zoephel A, Küpcü Z, Boerman O, Crommelin DJ, Wagner E, Kircheis R. Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm Res. 2000;17:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Treon SP, Raje N, Anderson KC. Immunotherapeutic strategies for the treatment of plasma cell malignancies. Semin Oncol. 2000;27:598-613. [PubMed] |
| 47. | Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Tanaka Y, Koido S, Chen D, Gendler SJ, Kufe D, Gong J. Vaccination with allogeneic dendritic cells fused to carcinoma cells induces antitumor immunity in MUC1 transgenic mice. Clin Immunol. 2001;101:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Scanlan MJ, Jäger D. Challenges to the development of antigen-specific breast cancer vaccines. Breast Cancer Res. 2001;3:95-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Brugger W, Brossart P, Scheding S, Stuhler G, Heinrich K, Reichardt V, Grünebach F, Bühring HJ, Kanz L. Approaches to dendritic cell-based immunotherapy after peripheral blood stem cell transplantation. Ann N Y Acad Sci. 1999;872:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Jäger E, Jäger D, Knuth A. Strategies for the development of vaccines to treat breast cancer. Recent Results Cancer Res. 1998;152:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Steinman RM. Dendritic cells and immune-based therapies. Exp Hematol. 1996;24:859-862. [PubMed] |
| 53. | Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Hermans IF, Moroni-Rawson P, Ronchese F, Ritchie DS. The emerging role of the dendritic cell in novel cancer therapies. N Z Med J. 1998;111:111-113. [PubMed] |
| 55. | Engleman EG. Dendritic cells: potential role in cancer therapy. Cytotechnology. 1997;25:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |