Published online Feb 15, 2003. doi: 10.3748/wjg.v9.i2.233
Revised: August 4, 2002
Accepted: August 29, 2002
Published online: February 15, 2003
AIM: Cancer gene therapy has received more and more attentions in the recent decade. Various systems of gene therapy for cancer have been developed. One of the most promising choices is the suicide gene. The product of thymidine kinase (TK) gene can convert ganciclovir (GCV) to phosphorylated GCV, which inhibits the synthesis of cell DNA, and then induces the cells to death. Cytokines play an important role in anti-tumor immunity. This experiment was designed to combine the TK gene and mIL-2/mGM-CSF genes to treat gastric cancer, and was expected to produce a marked anti-tumor effect.
METHODS: TK gene was constructed into the retroviral vector pLxSN, and the mIL-2 and mGM-CSF genes were inserted into the eukaryotic expressing vector pIRES. The gastric cancer cells were transfected by retroviral serum that was harvested from the package cells. In vitro study, the transfected gastric cancer cells were maintained in the GCV- contained medium, to assay the cell killing effect and bystander effect. In vivo experiment, retroviral serum and cytokines plasmid were transfected into tumor-bearing mice, to observe the changes of tumor volumes and survival of the mice.
RESULTS: In vitro experiment, 20% TK gene transduced cells could cause 70%-80% of total cells to death. In vivo results showed that there was no treatment effect in control group and TK/GCV could inhibit the tumor growth. The strongest anti-tumor effect was shown in TK+mIL-2+mGM-CSF group. The pathologic examination showed necrosis of the cancer in the treated groups.
CONCLUSION: TK/GCV can kill tumor cells and inhibit the tumor growth in vivo. IL-2 and GM-CSF strongly enhance the anti-tumor effect. Through the retrovirus and liposome methods, the suicide gene and cytokine genes are all expressed in the tissues.
- Citation: Guo SY, Gu QL, Zhu ZG, Hong HQ, Lin YZ. TK gene combined with mIL-2 and mGM-CSF genes in treatment of gastric cancer. World J Gastroenterol 2003; 9(2): 233-237
- URL: https://www.wjgnet.com/1007-9327/full/v9/i2/233.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i2.233
Gastric cancer is a common malignancy in China. However, all the efforts of conventional treatments including extended resection, radiation and chemotherapy have a little influence on the improvement of its survival. In searching for a new way to the treatment of such a malignant disease, the gene therapy was introduced and displayed its promising. One of the landmark discoveries is the application of suicide gene to cancer cells. It converted a nontoxic prodrug into a cell-killing compound. The herpes simplex virus type I thymidine kinase (HSV-tk) and the Escherichia coli cytosine deaminase (CD) was popularly used as transfected suicide gene.
The expressed products of these genes are enzymes, which can convert the nontoxic anti-ancer drugs into toxic ones, and disrupt the synthesis of target DNA. The product of TK gene can phosphalate the ganciclovir (GCV), and it was further phosphalated by endogenous kinase that leads to the formation of cytotoxic ganciclovir triphosphate. Interestingly, neighbor tumor cells that do not express the suicide gene were also killed in the presence of prodrug. This phenomenon is called the “bystander effect” [1-10].
Cytokines play important roles in the anti-tumor immune responses. IL-2 can activate the NK, LAK cells and CD8+ T lymphocytes. The activated CD8+ T lymphocytes can kill tumor cells directly. GM-CSF can promote the antigen presentation to macrophage and dendritic cells in the anti-tumor immune reaction[11-14].
The aim of this study was to boost the anti-tumor effect to achieve long- term survival and tumor eradication in model by the combination of TK/GCV with IL-2 and GM-CSF.
The retroviral vector pLxSN was purchased from the Genetech, the HSV-TK gene was provided by Dr. Bingya Liu. LacZ gene was purchased from Promega. MFC cell line was derived from the 615 murine carcinoma of proximal stomach, and obtained from the Drug Research Institute of Chinese Science Academy. PA317 cell and NIH3T3 cells were cultured in this laboratory. Ganciclovir was purchased from Shanghai Roche Company, DMEM from Gibco, and G 418 from Promega.
Vectors and cell lines The retroviral vector is pLxSN. TK gene was inserted into the multiple cloning site between EcoR I and BamH I, which was under the control of long terminal repeat (LTR), and the neomycin resistance gene was driven by an SV40 promoter. The report gene LacZ was inserted as same as TK gene. The murine IL-2 (Mil-2) and murine GM-CSF (mGM-CSF) were cloned from murine spleen tissue, and was confermed by DNA sequencing. They were inserted into multiple cloning site of the pIRES vector through the EcoR I and BamH I, and driven by the cytomegalovirus (CMV) promoter.
MFC cells were maintained in DMEM (Dubecco's modified essential medium), supplemented with 10% FBS (Hangzhou Sijiqing Biotech Company), 2 mM L-glutamine, 100 units/mL penicillin and 100 ug/mL streptomycin. PA317 cell was used as the packaging cell, and NIH 3T3 cell was used to assay the virus titre.
Packaging cells transfection, clone selection and supernatant preparation The retrovirus plasmids containing TK and LacZ gene were transfected into the PA317 packaging cell line by lipofectamine (Gibco). Clones were isolated by G418 selection. After 48hs of lipofection, the media was replaced by the media contain G418 (600 ug/mL). The media was changed every 3 d. Most cells died after 2 wk and the transfected cells survived. Culture and generate the selected anti-G418 cells. Collect virus suspension of four generations. To infect the NIH 3T3 cell with the virus suspension in different titres. Calculate the virus titres.
Infection of MFC gastric carcinoma cell line Infection was performed in suspension by a 30 min incubation of MFC cells with virus dilutions in 1 mL of PBS, supplemented with 4 ug/mL polybrene. To change the medium with DMEM which contained G418 48hs later, and repeated it every 3 d. Cells started to die after one week. The infected cells survived ultimately and formed cell clones.
1. In vitro sensitivity to GCV: Transfected MFC cells were planted in the 96 wells plate in 1 × 104/well. The medium containing various concentration of GCV. Cells were cultured for 7 d, and the survival rate of cells was counted by MTT method. The result was calculated by the following formula,
Survival rate = [(Value of experimental group - value of control group)/(The max value -value of control group)] × 100%
2. In vitro evaluation of the bystander effect: The transfected MFC cells were mixed with untransfected MFC cells at varying ratios, and planted in 96 wells plate at a density of 1 × 104/well. Cells were then cultured at 37 °C for 7 d in the present of 50 μg/mL GCV. The survival rate of cells was measured by MTT method.
The mouse MFC gastric cancer models were established by injecting of 5 × 105cells (in 100 μL saline) into the flanks of 60 6-week-old female 615 mice (the Animal Laboratory of the Institute of Drugs, Chinese Academy of Science). Five days late, when tumors became palpable, the mice were randomized in a blinded manner into 5 groups: the control group, TK, TK/GCV, TK/GCV+mIL-2, TK/GCV+mGM-CSF, and TK/GCV+mIL-2+mGM-CSF.
The test supernatant containing retrovirus was injected into tumors, 100 μL/time, twice a week, for 2weeks. The control group was injected with saline. The cytokine plasmids were transduced with liposome. The quantity of DNA was 50 μg/time/day and the volume ratio of DNA versus liposome was 5:1. The DNA/liposome mixture was injected for 4 consecutive days. Three days after the virus infection, the animals were treated with i.p. injection of GCV (500 ng/kg/d) or saline (control group). The treatment was maintained for 3 wk. Measure the longest radius (A) and the shortest radius (B) of tumors at every 5 d. The tumor volumes were calculated in mm3 using the formula 1/2AB2.
RNA from tissues of mice receiving TK and cytokine genes were extracted and examined by RT-PCR and agarose gel electrophoresis.
All the primers were designed by ourselve. The forward primer of TK gene was 5'GTGAATTCACAATGGCTT-CGTACCCCTGCCAT-3' and the reverse primer of TK gene was 5'-AGTGGATCCTCAGTTAGCCTCCCCCATCTCC-3' The forward primer of mβ-actin was 5'-TAGCGGGGTCACCCACAC-3' and the reverse primer of mβ-actin was 5'-CTAGAAGCACTTGCGGTGCACG-3'
The reaction condition of TK gene was denaturation in 95 °C for 1 min, annealing in 60 °C for 1 min, and extension in 72 °Cfor 3 min, for 39 cycles. The PCR conditions for β-actin were denaturation in 95 °C for 1 min, annealing in 55 °C for 1minute, and extension at 72 °C for 1 min, for 39 cycles. The products of RT-PCR were analyzed by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
To assess the expression of mIL-2 and mGM-CSF, the mRNA was extracted from the tumor surrounding tissues of mice and examined by RT-PCR. The forward primer of mIL-2 was 5'-TCGAATTCTGTACAGCATGCAGCTC-3' the reverse primer was 5'-TGGATCCGGTACATAGTTATTGAGGGC-3' The forward primer of mGM-CSF was 5'CGGAATTCAT-GTGGCTGCAGAAT-3' and the reverse primer was 5'-CGGAATTCTTCAGAGCTGGCCTG-3' The reaction condition was denaturation in 95 °C for 1 min, annealing in 55 °C for 55 s, and extension in 72 °C for 1 min, for 30 cycles. The products were analyzed by 1% agarose gel electrophoresis and visulized by ethidium bromide staining.
Samples of tumor and surrounding tissues were fixed with formalin for 24 h, wax embedded. Sections were obtained with a microtome, and stained with haematoxylin-eosin for histological analysis. The frozen samples were fixed with cold acetone for 10 min and immunostain with specific antibodies using peroxidase method to detect the expression of CD3+, CD4+and CD8+ in infiltrating cells. The sections were incubated for 15 min in phosphate buffered saline (PBS), 1% bovine serum albumin (BSA), and then overnight at 4 with monoclonal antibodies diluted in PBS/1% BSA.
The tumor volumes were performed using the variance analysis. P < 0.05 was considered to be statistically significant.
The TK gene retrovirus vector plasmid was transduced into packaging cells with lipofectamine and maintained for 5-7 d in culture medium containing G418600 mg/mL, and many cells started to death. After cultured for 2-3 wk, some adhesive cells formed cell clones contrasted with the dead cells.
The supernatants of every clone were collected and filtered after the cell clones were selected and expanded, the number of retroviral particles produced by the different cell clones was measured by NIH 3T3 cells. The maximum titer was 2 × 105 cfu/mL.
After infected by the virus supernatant, many MFC cells began to die. Some adhesive cells immerged 3 wk later, and formed cell clones.
MFC cells expressing TK gene were assayed for sensitivity to GCV. From the second day of culture with the medium containing GCV, the TK gene transfected cells began to die, and almost all the cells died at the seventh day. The untransfected cells in control group had no marked death.
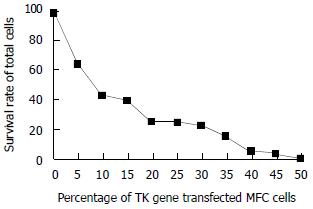
The TK gene transfected MFC cells expressing marked bystander effect. A few transfected cells can cause many co-cultured cells to death combined with GCV (50 u/mL), Twenty percent of the TK gene transfected cells could kill 70%-80% of total cells (Figure 1).
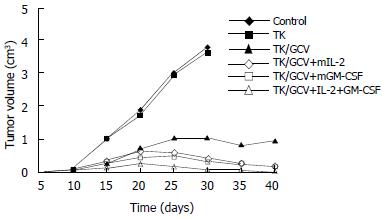
In vivo analyses of TK/GCV and cytokines were performed in 615 mice implanted with the mouse carcinoma MFC cell line in proximal stomach. The retrovirus supernatant was injected into the tumors, and the cytokine genes were injected into the tumor surrounding tissues as indicated in the “bterials and methods” There has no significant inhibition of tumor growth in control group although treated with peritoneum injection of GCV. The group of TK gene without use of GCV also had no inhibition effect on tumor growth. In the TK/GCV group, tumor growth was significantly suppressed (P < 0.01). In the animal groups treated with both TK/GCV and mIL-2 or mGM-CSF, there was a further significant reduction of the residual tumor size as compared to the group treated with TK/GCV (P < 0.05). There was further more dicrease of tumor size in the group of TK/GCV combined with both cytokines. The tumors diminished in 7 mice of this group (Figure 2).
There were great many tumor cells with mitoses in the sections of control group. The TK/GCV group showed lots of necrotic cells, and some of them accompanied by blee-ding. But active tumor cells could also be seen in this group. There was massive infiltration of inflammation cells surrounding the necrotic area of the tumor treated with TK + cytokine, but not in those area of animals treated with TK alone. Tumor cells diminished in most animals treated with TK/GCV+ mIL-2 + mGM-CSF. There were a few residue tumor tissues in part of these animals, but few mitoses phase can be seen, with great many of inflammatory cells.
Immunohistochemical analyses revealed that the infiltrates were mainly CD8+ lymphocytes in the tumor boundary area of animals treated with TK+mIL-2 or TK+ mIL-2 + mGM-CSF. The number of CD4+ lymphocytes was approximately equal in the TK + mGM-CSF and TK+mIL-2 + mGM-CSF groups.
By RT-PCR analyses, TK gene and cytokine genes all can be expressed in vivo by virus transfection or liposome transduction.
Transfer suicide genes into tumors has emerged as an attractive gene therapy for the selective elimination of cancer cells. The suicide genes encode non- mammalian enzymes that can convert nontoxic prodrugs into cellular toxic metabolites. The most widely used suicide gene is HSV-tk, which confers prodrug GCV into phosphorated GCV. The GCV monophosphate is further phosphorated by cellular kinase, forming GCV triphosphate, which inhibits cellular DNA synthesis and lead to cell death. The “dystander effect” caused by TK gene can strongly enhance its killing capacity[15-20]. Many researchers believe that necrosis of tumor cells is the mechanism of tumor killing effect caused by the metabolites of prodrugs, but the activated CTL can kill tumor cells as well. There also have many people think that apoptosis take an important role in the procession[21-24]. In our studies, necrosis was shown in the prodrug used tumor tissues, some of them with bleeding. This might be the vascular endothelials transfected by suicide genes. In vitro experiment showed that 20% gene tansfected cells rendered 80% of total cells to death. The mechanism of bystander effect has unclear. It has been hypothesized that the following factors may be concerned with the mechanism. (1) Gap junction: the toxic product of suicide gene was transferred from transfected cells into the surrounding untransfected ones[25-27]. Studies demonstrated that the bystander effect of TK gene was via the gap junction. The converted phosphorated GCV can get into the contact cells by gap junction, which needs the direct cell contact[28-33]; (2) Apoptosis: the apoptotic acetes that released by the transfected cells engulfed by the surrounding cells[34-37]; (3) Immune mechanism: tumor cells killed by TK/GCV can release tumor antigens. The tumor cell derived antigens were taken up by APCs (antigen presenting cells), and then presented to the CD4+ T lymphocytes. It in turn activated tumor-specific CD8+ cytolytic T cells. The immunohistochemistry shows tremendous aggregation of CD4+ and CD8+ lymphocytes surrounding the tumor tissue[38-42].
Chen et al[43] reported that cytokine gene IL-2 acted synergistically with the suicide gene to induce a systemic antitumor immunity. The immunity resulted in regression of local tumor and protection against distant site challenge of parental tumor cells. The antitumor immunity was attributed to IL-2 mediated activation and proliferation of CD8+ CTLs[43].
TK/GCV gene therapy led to death of the tumor cells. The tumor antigens were then available to the immune system, and might activate an anti-tumor immune response. The local expression of mGM-CSF enhanced the inflammatory response and antigen presentation. Expressed mIL-2 activated and enhanced the proliferation of T lymphocytes. Combination of mIL-2 with mGM-CSF can synergistically stimulate the anti-tumor immune response[44-48].
The experimental results confirmed that TK/GCV gene therapy could kill tumor cells markedly. If combined with mIL-2 and mGM-CSF genes, they could boost the anti- tumor reaction, and produce powerful anti-tumor effects.
Edited by Zhang JZ
| 1. | Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going. J Natl Cancer Inst. 1997;89:21-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 411] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Huber BE, Richards CA, Krenitsky TA. Retroviral-mediated gene therapy for the treatment of hepatocellular carcinoma: An innovative approach for cancer therapy. Proc Natl Acad Sci USA. 1991;88:8039-8043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 186] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The “bystander effect” : tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274-5283. [PubMed] |
| 4. | Fick J, Barker FG, Dazin P, Westphale EM, Beyer EC, Israel MA. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc Natl Acad Sci USA. 1995;92:11071-11075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Gore ME, Collins MK. Gene therapy for cancer. Eur J Cancer. 1994;30A:1047-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276-5281. [PubMed] |
| 7. | Moolten FL. Drug sensitivity (“ suicide”) genes for selective cancer chemotherapy. Cancer Gene Ther. 1994;1:279-287. [PubMed] |
| 8. | Mulligan RC. The basic science of gene therapy. Science. 1993;260:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1143] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 9. | Marcel T, Grausz JD. The TMC Worldwide Gene Therapy Enrollment Report, end 1996. Hum Gene Ther. 1997;8:775-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1074] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 11. | Freeman SM, Ramesh R, Marrogi AJ. Immune system in suicide-gene therapy. Lancet. 1997;349:2-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Mahvi DM, Burkholder JK, Turner J, Culp J, Malter JS, Sondel PM, Yang NS. Particle-mediated gene transfer of granulocyte-macrophage colony-stimulating factor cDNA to tumor cells: implications for a clinically relevant tumor vaccine. Hum Gene Ther. 1996;7:1535-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Kim TS, Cohen EP. Interleukin-2-secreting mouse fibroblasts transfected with genomic DNA from murine melanoma cells prolong the survival of mice with melanoma. Cancer Res. 1994;54:2531-2535. [PubMed] |
| 14. | Cao GW, Gao J, Du P, Qi ZT, Kong XT. Construction of retroviral vectors to induce a strong expression of human class I interferon gene in human hepatocellular carcinoma cells in vitro. China Natl J New Gastroenterol. 1997;3:139-142. |
| 15. | Wei MX, Bougnoux P, Sacré-Salem B, Peyrat MB, Lhuillery C, Salzmann JL, Klatzmann D. Suicide gene therapy of chemically induced mammary tumor in rat: Efficacy and distant bystander effect. Cancer Res. 1998;58:3529-3532. [PubMed] |
| 16. | Boucher PD, Ruch RJ, Shewach DS. Differential ganciclovir-mediated cytotoxicity and bystander killing in human colon carcinoma cell lines expressing herpes simplex virus thymidine kinase. Hum Gene Ther. 1998;9:801-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Namba H, Tagawa M, Iwadate Y, Kimura M, Sueyoshi K, Sakiyama S. Bystander effect-mediated therapy of experimental brain tumor by genetically engineered tumor cells. Hum Gene Ther. 1998;9:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Su H, Lu R, Chang JC, Kan YW. Tissue-specific expression of herpes simplex virus thymidine kinase gene delivered by adeno-associated virus inhibits the growth of human hepatocellular carcinoma in athymic mice. Proc Natl Acad Sci USA. 1997;94:13891-13896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Sturtz FG, Waddell K, Shulok J, Chen X, Caruso M, Sanson M, Snodgrass HR, Platika D. Variable efficiency of the thymidine kinase/ganciclovir system in human glioblastoma cell lines: implications for gene therapy. Hum Gene Ther. 1997;8:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Chen CY, Chang YN, Ryan P, Linscott M, McGarrity GJ, Chiang YL. Effect of herpes simplex virus thymidine kinase expression levels on ganciclovir-mediated cytotoxicity and the “bystander effect”. Hum Gene Ther. 1995;6:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | McMasters RA, Saylors RL, Jones KE, Hendrix ME, Moyer MP, Drake RR. Lack of bystander killing in herpes simplex virus thymidine kinase-transduced colon cell lines due to deficient connexin43 gap junction formation. Hum Gene Ther. 1998;9:2253-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Touraine RL, Vahanian N, Ramsey WJ, Blaese RM. Enhancement of the herpes simplex virus thymidine kinase/ganciclovir bystander effect and its antitumor efficacy in vivo by pharmacologic manipulation of gap junctions. Hum Gene Ther. 1998;9:2385-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann JL, Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA. 1993;90:7024-7028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 265] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Tanaka T, Kanai F, Okabe S, Yoshida Y, Wakimoto H, Hamada H, Shiratori Y, Lan K, Ishitobi M, Omata M. Adenovirus-mediated prodrug gene therapy for carcinoembryonic antigen-producing human gastric carcinoma cells in vitro. Cancer Res. 1996;56:1341-1345. [PubMed] |
| 25. | Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93:1831-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 304] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Trinh QT, Austin EA, Murray DM, Knick VC, Huber BE. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808-4812. [PubMed] |
| 27. | Rogulski KR, Kim JH, Kim SH, Freytag SO. Glioma cells transduced with an Escherichia coli CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and radiosensitivity. Hum Gene Ther. 1997;8:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Denning C, Pitts JD. Bystander effects of different enzyme-prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum Gene Ther. 1997;8:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Yang L, Chiang Y, Lenz HJ, Danenberg KD, Spears CP, Gordon EM, Anderson WF, Parekh D. Intercellular communication mediates the bystander effect during herpes simplex thymidine kinase/ganciclovir-based gene therapy of human gastrointestinal tumor cells. Hum Gene Ther. 1998;9:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Vile RG, Nelson JA, Castleden S, Chong H, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228-6234. [PubMed] |
| 31. | Mullen CA, Coale MM, Lowe R, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994;54:1503-1506. [PubMed] |
| 32. | Yang Y, Nunes FA, Berencsi K, Furth EE, GönczöL E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407-4411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1163] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 33. | Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004-2015. [PubMed] |
| 34. | Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: A negative selection system. Proc Natl Acad Sci USA. 1992;89:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 332] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91:8302-8306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 296] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Consalvo M, Mullen CA, Modesti A, Musiani P, Allione A, Cavallo F, Giovarelli M, Forni G. 5-Fluorocytosine-induced eradication of murine adenocarcinomas engineered to express the cytosine deaminase suicide gene requires host immune competence and leaves an efficient memory. J Immunol. 1995;154:5302-5312. [PubMed] |
| 37. | Plautz GE, Yang ZY, Wu BY, Gao X, Huang L, Nabel GJ. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci USA. 1993;90:4645-4649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 186] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Ilsley DD, Lee SH, Miller WH, Kuchta RD. Acyclic guanosine analogs inhibit DNA polymerases alpha, delta, and epsilon with very different potencies and have unique mechanisms of action. Biochemistry. 1995;34:2504-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the 'bystander effect': A cytokine cascade. Exp Hematol. 1996;24:829-838. [PubMed] |
| 40. | Kianmanesh AR, Perrin H, Panis Y, Fabre M, Nagy HJ, Houssin D, Klatzmann D. A “distant” bystander effect of suicide gene therapy: regression of nontransduced tumors together with a distant transduced tumor. Hum Gene Ther. 1997;8:1807-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Wolff G, Körner IJ, Schumacher A, Arnold W, Dörken B, Mapara MY. Ex vivo breast cancer cell purging by adenovirus-mediated cytosine deaminase gene transfer and short-term incubation with 5-fluorocytosine completely prevents tumor growth after transplantation. Hum Gene Ther. 1998;9:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Addison CL, Braciak T, Ralston R, Muller WJ, Gauldie J, Graham FL. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci USA. 1995;92:8522-8526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Chen SH, Kosai K, Xu B, Pham-Nguyen K, Contant C, Finegold MJ, Woo SL. Combination suicide and cytokine gene therapy for hepatic metastases of colon carcinoma: sustained antitumor immunity prolongs animal survival. Cancer Res. 1996;56:3758-3762. [PubMed] |
| 44. | Sobol RE, Shawler DL, Carson C, Van Beveren C, Mercola D, Fakhrai H, Garrett MA, Barone R, Goldfarb P, Bartholomew RM. Interleukin 2 gene therapy of colorectal carcinoma with autologous irradiated tumor cells and genetically engineered fibroblasts: A Phase I study. Clin Cancer Res. 1999;5:2359-2365. [PubMed] |
| 45. | Palù G, Cavaggioni A, Calvi P, Franchin E, Pizzato M, Boschetto R, Parolin C, Chilosi M, Ferrini S, Zanusso A. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: A pilot study in humans. Gene Ther. 1999;6:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Gambotto A, Tüting T, McVey DL, Kovesdi I, Tahara H, Lotze MT, Robbins PD. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Cancer Gene Ther. 1999;6:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Saffran DC, Horton HM, Yankauckas MA, Anderson D, Barnhart KM, Abai AM, Hobart P, Manthorpe M, Norman JA, Parker SE. Immunotherapy of established tumors in mice by intratumoral injection of interleukin-2 plasmid DNA: induction of CD8+ T-cell immunity. Cancer Gene Ther. 1998;5:321-330. [PubMed] |
| 48. | Shi FS, Weber S, Gan J, Rakhmilevich AL, Mahvi DM. Granulocyte-macrophage colony-stimulating factor (GM-CSF) secreted by cDNA-transfected tumor cells induces a more potent antitumor response than exogenous GM-CSF. Cancer Gene Ther. 1999;6:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |