Published online Dec 15, 2003. doi: 10.3748/wjg.v9.i12.2681
Revised: July 26, 2003
Accepted: September 13, 2003
Published online: December 15, 2003
AIM: To investigate the systemic availability of budesonide in a patient with Child A cirrhosis due to autoimmune hepatitis (AIH) and primary hepatocellular carcinoma, who developed serious side effects.
METHODS: Serum levels of budesonide, 6β-OH-budesonide and 16α-OH-prednisolon were measured by HPLC/MS/MS; portosystemic shunt-index (SI) was determined by 99mTc nuclear imaging. All values were compared with a matched control patient without side effects.
RESULTS: Serum levels of budesonide were 13-fold increased in the index patient. The ratio between serum levels of the metabolites 6β-OH-budesonide and 16α-OH-prednisolone, respectively, and serum levels of budesonide was diminished (1.0 vs. 4.0 for 6β-OH-budesonide, 4.2 vs. 10.7 for 16α-OH-prednisolone). Both patients had portosystemic SI (5.7% and 3.1%) within the range of healthy subjects.
CONCLUSION: Serum levels of budesonide vary up to 13-fold in AIH patients with Child A cirrhosis in the absence of relevant portosystemic shunting. Reduced hepatic metabolism, as indicated by reduced metabolite-to-drug ratio, rather than portosystemic shunting may explain systemic side effects of this drug in cirrhosis.
- Citation: Geier A, Gartung C, Dietrich CG, Wasmuth HE, Reinartz P, Matern S. Side effects of budesonide in liver cirrhosis due to chronic autoimmune hepatitis: influence of hepatic metabolism versus portosystemic shunts on a patient complicated with HCC. World J Gastroenterol 2003; 9(12): 2681-2685
- URL: https://www.wjgnet.com/1007-9327/full/v9/i12/2681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i12.2681
Autoimmune hepatitis is a chronic necroinflammatory liver disorder of unknown etiology associated with interface hepatitis, hypergammaglobulinemia and circulating autoantibodies, which was first described by Waldenström in 1950[1] and termed “autoimmune hepatitis” by the International Autoimmune Hepatitis Group in 1992[2]. The identification and characterization of serum autoantibodies led to differentiation of three different types characterized by antinuclear antibodies (type I), liver-kidney microsomal antibodies (type II) and soluble liver antigen (type III)[3,4]. The distinction between autoimmune hepatitis and other autoimmune liver diseases like primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) is based on characteristic clinical, histological, biochemical and immunological features but overlap and variant syndromes may occur[5].
Primary hepatocellular carcinoma (HCC) is thought to be a consequence of the progression from chronic hepatitis to cirrhosis, although this sequence appears to be rare in autoimmune hepatitis[6]. In many patients who develop HCC hepatitis C virus infection has been found to be a complicating condition[7]. However, HCC also occurs in the absence of risk factors like hepatitis C infection and corticosteroid therapy[8].
Autoimmune hepatitis is generally responsive to corticosteroid therapy despite its striking heterogeneity[1]. Corticosteroids alone or in combination with azathioprine are the treatment of choice and result in remission induction in over 80% of patients[9,10]. Early clinical trials with prednisolone documented improvement of liver function tests, amelioration of symptoms and prolonged survival, thereby establishing corticosteroids as the "standard therapy"[11-13]. Azathioprine has no role in inducing remission but may be used for maintenance of remission induced by initial corticosteroid therapy or their dose reduction in combination therapy[9]. The potential usefullness of other immunosuppressives like cyclosporine and tacrolimus has been shown but has not yet been clinically established[9]. Since about 70% of patients with AIH require lifelong immunosuppressive therapy, long-term use of corticosteroids is often accompanied by numerous systemic side effects, such as osteoporosis, diabetes mellitus, systemic hypertension, psychiatric disorders and altered steroid metabolism[14].
The search for new corticosteroids with a more favourable risk-to-benefit ratio led to the discovery of a new class of corticosteroids with high receptor affinity and particularly high first-pass effect in the liver resulting in lower systemic side effects. Budesonide, a nonhalogenated glucocorticoid derivative of the second generation exhibits a receptor affinity 15-20 times that of prednisolone and a 90% first-pass metabolism in healthy liver[9]. Two major metabolites, 6β-OH-budesonide and 16α-OH-prednisolone, have been identified which lack glucocorticoid activity making the original compound virtually devoid of systemic side effects[15]. Budesonide has been evaluated in patients with primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) at a dose of 9 mg/day with contradicting results concerning additional benefit to the standard therapy with ursodeoxycholic acid (UDCA)[16-19]. In autoimmune hepatitis only two small uncontrolled trials with a limited number of patients and rather disappointing results have been published[20,21]. In one study including 10 patients who were dependent on continuous treatment to prevent exacerbation budesonide failed to induce clinical and biochemical remission in 7 patients who either deteriorated or became drug intolerant[21]. In a second study with 13 patients budesonide has been found to lower transaminase levels significantly while causing a low frequency of systemic side effects and only a marginal reduction in plasma cortisol in noncirrhotic patients[20]. However, the majority of patients did not reach full biochemical remission defined as normal ALT values. Patients with cirrhosis experienced higher serum cortisol levels and a partial suppression of the hypothalamic-pituitary-adrenal (HPA) axis suggestive for at least a latent systemic activity of budesonide[20].
An explanation for a variable and so far unpredictable extent of systemic side effects in patients with early liver disease treated with budesonide may be either a reduced metabolic function or the presence of latent portosystemic shunts bypassing the liver. However, no data are presently available on the metabolism of budesonide in cirrhotic livers and the incidence of side effects in these patients. Thus, there are no recommendations for therapy with budesonide in cirrhotic patients with AIH to date.
To our knowledge, treatment of patients with coexisting HCC or other malignancies has so far not been reported in literature. Systemic immunosuppression exposes such patients to an increased risk of tumor recurrance after curative surgical treatment of the malignant disorder. A risk stratification for systemic effects of budesonide by assessment of the portosystemic shunt-index (SI) and the metabolic activity of the cirrhotic liver is of major importance in these patients. We report the first case of successfull treatment of a patient with AIH after resection of a HCC and show a predominance of hepatic metabolism over portosystemic shunting on sytemic availability and side effects of budesonide in this patient with Child A cirrhosis.
A 65-year-old caucasian woman (patient 1) with a 27-year history of unclassified chronic hepatitis was referred to our liver unit with a newly diagnosed liver mass during regular ultrasound screening. She experienced icteric episodes 27 and 14 years ago and a known asymptomatic cholecystolithiasis. Physical examination of the anicteric patient was unremarkable despite liver enlargement without a palpable mass. Clinical chemistry on admission showed mild signs of hepatic inflammation (AST 24 U/L, GGT 53 U/L) without elevation of further liver function tests or pancreatic enzymes. Biochemical tests revealed type I autoimmune hepatitis with antinuclear (titer 1:320), anticytoplasmatic (titer 1:320) and anti-smooth muscle antibodies (titer 1:640) as well as elevated gamma-globulins (29% of total proteins, IgG 25.7 g/L). Quantification of alpha-1-antitrypsin, ceruloplasmin, serum iron, ferritin, transferrin saturation, serum copper and urinary copper excretion were within normal limits, and serologic tests for anti-HAV, anti-HBs, anti-HBc, HBs-antigen and anti-HCV were all negative. On ultrasound the liver appeared cirrhotic with a 4.5 × 3.9 cm mass in the right lobe. Computed tomography (CT) depicted a 4 cm mass in segment VI of the right lobe of the liver with contrast enhancement. Alpha-1-fetoprotein (AFP) was not elevated. A CT-guided Tru-cut biopsy confirmed the diagnosis of a primary HCC in a cirrhotic liver. Tumor staging revealed no signs of metastasis in thoracic CT, 19fluor deoxyglucose positron emission tomography. Preoperative staging of the liver disease led to a grade A cirrhosis according to the Child-Pugh classification. Endoscopy of the upper GI tract did not detect obvious varices of the esophagus and gastric fundus.
The patient underwent atypical resection of segment VI liver without further complications. Follow-up in our liver outpatient clinic showed functional liver tests at the preoperative level with mild AST and GGT elevation. Three months after resection of the HCC, transaminase levels sharply increased over a period of 4 months with peak transaminases of ALT 247 U/L and AST 312 U/L. No signs of tumor recurrance were detectable by abdominal CT and AFP levels. In order to prevent further progression of the Child A cirrhosis, we initiated a therapy with budesonide which was favoured over systemic immunosuppressive treatment with respect to the antecipated lower risk of tumor recurrance. Transaminase levels dropped rapidly to the normal range during a daily dose of 9 mg budesonide. Remission was achieved within two months after starting budesonide, but systemic side effects such as facial swelling, leg edemas and weight gain occurred. To minimize these adverse effects and systemic immunosuppression, we tapered the dose to 6 mg/day for maintenance therapy. Follow-up for potential tumor recurrance with corticosteroid therapy revealed normal CT-scans and AFP levels so far. Almost four years after tumor resection, the patient is still in remission in regard to AIH at a daily dose of 6 mg budesonide. In this index patient we assessed the determinants of systemic effects of budesonide in comparison with another AIH patient matched for age, sex and functional Child-Pugh stage (patient 2, caucasian female, 67 years of age, Child A cirrhosis diagnosed by liver biopsy) with steroid dependency, no apparent varices) and historic healthy controls.
Levels of budesonide, 6β-OH-budesonide and 16α-OH-prednisolone were determined in serum 3 hours (allowance ± 60 minutes) after intake by a validated HPLC/MS/MS assay. Serum was extracted by solid phase extraction and the reconstituted extract was analyzed by HPLC/MS/MS on a Micromass Quatro LC using reversed phase chromatography and the negative electrospray ionization mode. The limit of detection was defined as 100 pg/ml for all analytes. All determinations have been performed by H.W. Moellmann (Medical Clinic Bergmannsheil, Bochum, Germany) and funded by Falk Pharma (Freiburg i.B., Germany). Falk Pharma had no involvement in analysis, interpretation and publication of the data.
Basal cortisol levels and cortisol levels in response to 100 µg corticotropine releasing hormone (CRH) were measured with routine clinical chemistry. Both tests were performed at 8 A. M. and stimulated cortisol levels were determined over 90 minutes after intravenous application of CRH.
Portosystemic SI was determined by nuclear imaging after rectal application of 99mTechnetium pertechnetate (Tc) as described[22]. Briefly, the rectum was emptied by administration of a laxative and a polyethylene tube was inserted. After positioning of a large-field scintillation camera over the patient 340 MBq of 99mTc pertechnetate was given over the tube and time-activity curves for the areas of the liver and heart were obtained. After normalization portosystemic SI was calculated from the activity of the liver and heart.
Cortisol and portosystemic shunt-index was measured at the time of the first serum analysis on budesonide and metabolites. All measurements were determined with informed consent by both patients.
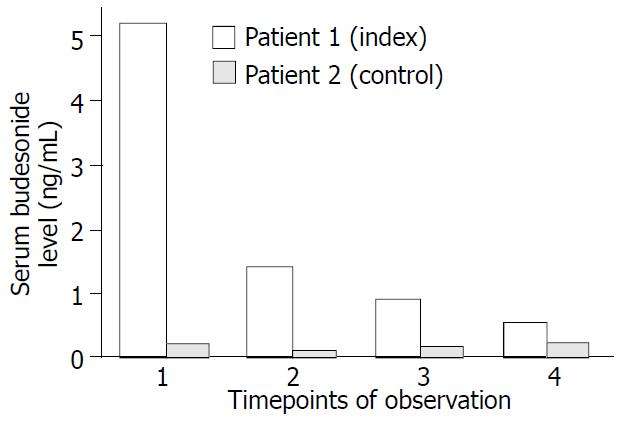
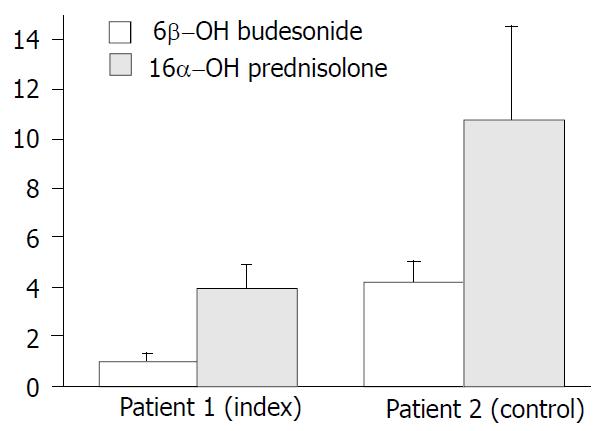
Serum budesonide levels were determined 3 h after intake of 6 mg at 4 different time points during five months (Figure 1). At all time points serum budesonide levels were markedly elevated in the index case (mean 1.9 ng/ml) when compared with the control patient (mean budesonide level 0.15 ng/ml) and historic healthy controls without liver disease (0.4-0.7 ng/ml)[23]. Systemic availability in patient 2 was remarkably lower even with a higher dose of 9 mg budesonide. However, serum levels of this control patient was in close comparison with the historic healthy controls, further supporting a potential clinical relevance of the elevated serum budesonide levels in the index patient. To determine whether elevated serum budesonide levels resulted from impaired hepatic metabolism, 6β-OH-budesonide and 16α-OH-prednisolone as its major inactive metabolites were measured in both patients (Figure 2). Although both metabolites were detectable in both the index and control patient, the mean ratio of metabolites to drug serum levels were significantly lower for both 6β-OH-budesonide (1.0- vs. 4.0-fold) and 16α-OH-prednisolone (4.2- vs. 10.7-fold) in the index compared with the control patient (Figure 2).
To further determine the potential systemic effects of budesonide, serum cortisol levels were measured at baseline and after stimulation by 100 µg corticotropine releasing hormone (CRH). Whereas basal and stimulated serum cortisol levels were within the normal range in the control patient, the index patient showed marked suppression of serum cortisol [ < 6 nmol/L (normal range 119-618 nmol/l)] and no significant stimulation by CRH within 90 min after application (maximum level 63 nmol/L at 60 min).
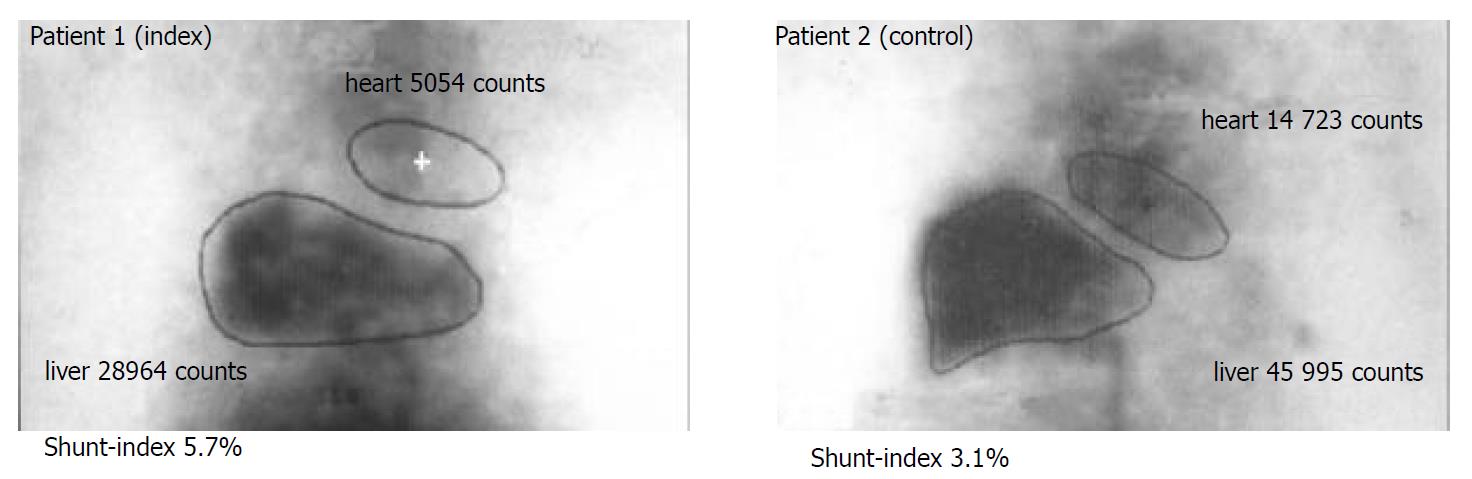
To differentiate whether elevated serum budesonide levels were caused by increased portosystemic shunting due to liver cirrhosis, portal hemodynamics were assessed by per-rectal portal scintigraphy with 99mTc pertechnetate in both patients. In both the index and the control patient measurement of the portosystemic shunt index was 5.7% and 3.1%, respectively, which was in the range of healthy subjects (median 4.1%; 25th percentile 2.8; 75th percentile 6.3%)[22]. Thus, these data confirmed that no significant portosystemic shunting occurred in both patients (Figure 3).
Autoimmune hepatitis is currently treated with systemic administration of prednisolone, which has been shown to improve liver function tests and prolong survival[11-13]. These first-generation glucocorticoids may cause marked side effects in long-term therapy. In order to minimize the systemic bioavailability, a new class of corticosteroids with greater topical anti-inflammatory activity and particularly high first-pass effect in healthy liver was synthesized[24]. Budesonide, a member of this second generation, has a 90% first pass metabolism in the healthy liver and its metabolites are virtually devoid of systemic side effects[9]. Therefore, it does not reduce peripheral cortisol levels as a measure of systemic effect on adrenal function to the same extent as prednisolone in noncirrhotic patients[24]. However, in a double-blind crossover study in healthy volunteers ileal release budesonide had a greater effect on plasma cortisol levels compared with placebo[25]. In patients with Crohn’s disease budesonide reduced the median plasma cortisol concentrations at doses of 9-15 mg/day, whereas median cortisol values in the group given 3 mg/day were not different from placebo[26].
In patients with liver disease, systemic side effects are variable. In patients with primary biliary cirrhosis treated with 9 mg budesonide per day (in addition to UDCA) changes in bone mineral density were not significantly different as compared with pretreatment data and placebo over 2 years in the study by Leuschner et al[16], whereas Angulo and coworkers found a significant loss of bone mass with the same treatment regimen over one year[17]. This difference may be related to the number of patients with PBC stage IV of disease who experienced a significantly greater loss of bone mass in the Angulo study compared with non-cirrhotic patients. Leuschner and coworkers enrolled only patients of PBC stages I to III in this study. Similar findings were obtained in patients with primary sclerosing cholangitis in whom budesonide appears to be of minimal if any benefit and is associated with a significant worsening of osteoporosis[18,19]. Although the drug is considered to be a promising candidate for treatment, only two small uncontrolled studies on budesonide in patients with autoimmune hepatitis have been published to date[20,21]. In parallel with findings in PBC and PSC therapeutic efficacy of budesonide on AIH appeared to be limitied and systemic side effects shown as decreased plasma cortisol levels were noted predominantly in those patients with cirrhosis[15,20]. Although there are no published data on a possible decrease in first pass hepatic metabolism in patients with cirrhosis and consecutive higher incidence of systemic adverse effects, indirect findings such as increased osteoporosis in budesonide-treated patients with more advanced liver disease support this hypothesis[17]. This could be due to reduced hepatic metabolism in the cirrhotic liver or, alternatively, due to increased spontaneous portosystemic shunting in these patients who may have a substantial bypass of the liver.
Portosystemic collaterals develop as chronic hepatitis and cirrhosis progress. Several methods for measurement of portosystemic shunting have been established, of which radio-isotopic imaging after per-rectal 99mTc administration with determination of the heart-liver ratio (shunt index) is most common[22,27-30]. A cross sectional study using this method has shown an increasing portal shunt index (SI) with progression of various liver diseases[22]. In this study, healthy controls and patients with chronic hepatitis showed shunt indices with medians between 5.9% and 10%, respectively. Patients with cirrhosis without esophageal varices had only moderatly increased portosystemic shunt indices with a median of 15% (25th percentile 9%, 75th percentile 28%) whereas those cirrhotics with varices appeared to have dramatically increased shunting with a median SI of 70% (25th percentile 52%, 75th percentile 82%).
Hepatic metabolism and the extent of the portosystemic shunting largely affects the bioavailability of drugs with high first-pass metabolism such as budesonide. To date, there are no data about systemic availability of the drug and its determinants in patients with advanced liver disease. To address this question and to distinguish between reduced hepatic metabolism and portosystemic shunting as the cause of higher bioavailability, we determined the serum levels of budesonide, its two major metabolites and the portal shunt index in two patients. The index patient 1 was compared with an age and sex matched AIH-patient with the same stage of cirrhosis but absent systemic effects as control. Serum levels of budesonide were largely elevated in the index case (1.9 ± 2.1 ng/mL, n = 4) compared with the control patient 2 (0.15 ± 0.07 ng/mL, n = 4) and historically healthy controls without liver disease (0.4-0.7 ng/mL)[23] (Figure 1). Although 6β-OH-budesonide and 16α-OH-prednisolone serum levels were also elevated 3-fold and 4.4-fold in the index patient as against patient 2, we detected 4-fold decreased metabolite-to-drug ratios for both 6β-OH-budesonide and 16α-OH-prednisolone in the index patient 1 compared with the control patient 2 (Figure 2). Both patients had similar shunt indices which were in the range of healthy subjects (Figure 3). These data demonstrate that an increased systemic bioavailability of budesonide may be rather due to a reduced hepatic metabolizing capacity in patients with early stages of liver disease than portosystemic shunting of an undetectable volume.
In these patients with coexisting malignancies, there is major concern about corticosteroid-related systemic immunosuppression which imposes a substantial risk of recurrance or progression of malignancy on these patients. To our knowledge, there is no report available on steroid therapy in a patient with autoimmune hepatitis and coexisting hepatic malignancy. The present case is the first reported patient with successfull treatment of AIH complicated by HCC. The substantial inflammatory activity in this patient, who had already developed liver cirrhosis, made an anti-inflammatory therapy unevitable to halt progression. In this complex situation between progression to end stage cirrhosis and recurrance of the carcinoma, budesonide seems to be the only therapeutic alternative to minimize both risks. Although low bioavailability and high first pass metabolism of budesonide are observed in healthy volunteers, systemic availability in patients with liver cirrhosis seems to be associated with decreased hepatic metabolism after exclusion of significant portosystemic shunting (Figure 3). In order to minimize systemic immunosuppression with a concommitant risk of recurrant malignancy, we tapered the daily dose from 9 mg to 6 mg per day after systemic side effects appeared. Our present study identified increased systemic budesonide levels due to reduced hepatic metabolism as a risk factor of tumor recurrance in this patient. Therefore, we closely monitored the recurrance of the HCC in short intervals by ultrasound, computed tomography and AFP levels without any signs of recurrant malignancy under budesonide for 36 months.
Whether budesonide becomes standard therapy in autoimmune hepatitis remains to be determined in larger clinical trials. In patients with concommitant malignant disorders, this drug may have the best benefit-to-risk ratio of all immunosuppressants and may be helpful even in patients with reduced hepatic metabolism due to liver cirrhosis. However, these patients have a substantial risk of systemic side effects even in the absence of detectable portosystemic shunting and need to be monitored carefully.
Edited by Ma JY
| 1. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 1996;334:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 664] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Manns MP, Strassburg CP. Autoimmune hepatitis: clinical challenges. Gastroenterology. 2001;120:1502-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Strassburg CP, Manns MP. Autoantibodies and autoantigens in autoimmune hepatitis. Semin Liver Dis. 2002;22:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1986] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 6. | Wang KK, Czaja AJ. Hepatocellular carcinoma in corticosteroid-treated severe autoimmune chronic active hepatitis. Hepatology. 1988;8:1679-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ryder SD, Koskinas J, Rizzi PM, McFarlane IG, Portmann BC, Naoumov NV, Williams R. Hepatocellular carcinoma complicating autoimmune hepatitis: role of hepatitis C virus. Hepatology. 1995;22:718-722. [PubMed] |
| 8. | Watanabe M, Moritani M, Hamamoto S, Uchida Y, Ishihara S, Adachi K, Kinoshita Y. Hepatocellular carcinoma complicating HCV-negative autoimmune hepatitis without corticosteroid therapy. J Clin Gastroenterol. 2000;30:445-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Heneghan MA, McFarlane IG. Current and novel immunosuppressive therapy for autoimmune hepatitis. Hepatology. 2002;35:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Czaja AJ. Treatment of autoimmune hepatitis. Semin Liver Dis. 2002;22:365-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820-833. [PubMed] |
| 12. | Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 338] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 302] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 14. | Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune hepatitis. J Hepatol. 2000;32:181-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Ryrfeldt A, Andersson P, Edsbäcker S, Tönnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;122:86-95. [PubMed] |
| 16. | Leuschner M, Maier KP, Schlichting J, Strahl S, Herrmann G, Dahm HH, Ackermann H, Happ J, Leuschner U. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 165] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Angulo P, Jorgensen RA, Keach JC, Dickson ER, Smith C, Lindor KD. Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology. 2000;31:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | van Hoogstraten HJ, Vleggaar FP, Boland GJ, van Steenbergen W, Griffioen P, Hop WC, van Hattum J, van Berge Henegouwen GP, Schalm SW, van Buuren HR. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am J Gastroenterol. 2000;95:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2333-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 94] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Czaja AJ. Drug therapy in the management of type 1 autoimmune hepatitis. Drugs. 1999;57:49-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Shiomi S, Sasaki N, Habu D, Takeda T, Nishiguchi S, Kuroki T, Tanaka T, Ochi H. Natural course of portal hemodynamics in patients with chronic liver diseases, evaluated by per-rectal portal scintigraphy with Tc-99m pertechnetate. J Gastroenterol. 1998;33:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Mollmann HW, Hochhaus G, Tromm A, Froehlich P, Mollmann AC, Krieg M, Weiser H, Derendorf H, Barth J. Pharmacokinetics and pharmacodynamics of budesonide pH-modified release capsules. In: Mollmann HW, May B, eds. Glucocorticoid therapy in chronic inflammatory bowel disease - from basic principles to rational therapy. Dordrecht, Boston, London: Kluwer Academic Publishers. 1996;107-120. |
| 24. | Spencer CM, McTavish D. Budesonide. A review of its pharmacological properties and therapeutic efficacy in inflammatory bowel disease. Drugs. 1995;50:854-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Edsbäcker S, Nilsson M, Larsson P. A cortisol suppression dose-response comparison of budesonide in controlled ileal release capsules with prednisolone. Aliment Pharmacol Ther. 1999;13:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, Nilsson LG, Persson T. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994;331:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 348] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Shiomi S, Kuroki T, Kurai O, Kobayashi K, Ikeoka N, Monna T, Ochi H. Portal circulation by technetium-99m pertechnetate per-rectal portal scintigraphy. J Nucl Med. 1988;29:460-465. [PubMed] |
| 28. | Shiomi S, Kuroki T, Ueda T, Takeda T, Ikeoka N, Nishiguchi S, Nakajima S, Kobayashi K, Ochi H. Clinical usefulness of evaluation of portal circulation by per rectal portal scintigraphy with technetium-99m pertechnetate. Am J Gastroenterol. 1995;90:460-465. [PubMed] |
| 29. | D'Arienzo A, Celentano L, Cimino L, Panarese A, Lancia C, Vergara E, Castaldo G, Oriani G, Squame G, Budillon G. Per-rectal portal scintigraphy with technetium-99m pertechnetate for the early diagnosis of cirrhosis in patients with chronic hepatitis. J Hepatol. 1992;14:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Wang JY, Chen SL, Chen FZ, Xu WG, Hu DC, Chen XF, Jin G, Liu HY. A non-invasive method for evaluating cirrhotic portal hypertension by administration of 99mTc-MIBI per rectum. J Gastroenterol Hepatol. 1995;10:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |