Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2356
Revised: June 12, 2003
Accepted: June 19, 2003
Published online: October 15, 2003
AIM: To study and compare the difference of activation-induced cell death (AICD) in peripheral blood T-lymphocytes (PBL-Ts) from patients with chronic hepatitis B (CHB) and the normal people in vitro, and to explore the role of AICD in chronic hepatitis B virus (HBV) infection and the pathogenesis of CHB.
METHODS: Twenty-five patients and fourteen healthy people were selected for isolation of PBL-Ts. During cultivation, anti-CD3 mAb, PMA and ionomycin were used for AICD of PBL-Ts. AICD ratio of PBL-Ts was detected with TdT-mediated dUTP nick end labeling and assessed by flow cytometry.
RESULTS: When induced with anti-CD3, PMA and ionomycin in vitro, AICD ratio of PBL-Ts from CHB patients was significantly higher than that from healthy control (17.24 ± 1.21 vs. 6.63 ± 1.00, P < 0.01) and that from CHB patients without induction (17.24 ± 1.21 vs. 9.88 ± 1.36, P < 0.01). There was a similar AICD ratio of PBL-Ts between induction group and without induction group, but no difference was found before and after induction in healthy control. The density of INF-γ in culture media of induction groups of CHB was lower than that of other groups (P < 0.01). There was no difference between these groups in density of IL-10 (P > 0.05).
CONCLUSION: When induced during cultivation in vitro, PBL-Ts from CHB have AICD very commonly. This phenomenon has a potentially important relation with pathogenesis of CHB and chronicity of HBV infection.
- Citation: Hou CS, Wang GQ, Lu SL, Yue B, Li MR, Wang XY, Yu JW. Role of activation-induced cell death in pathogenesis of patients with chronic hepatitis B. World J Gastroenterol 2003; 9(10): 2356-2358
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2356
Currently, the exact pathogenesis of chronic hepatitis B (CHB) and the reason of chronic hepatitis B virus (HBV) infection are still not completely understood. Activation-induced cell death (AICD) is related with lymphocytes decrease and functional defect. This phenomenon can cause decrease of immune clearance. Alloreactive T cells can effectively be depleted from allogeneic T cells by induction of AICD to prevent graft-versus-host disease[1]. AICD is essential for the function, growth and differentiation of T-lymphocytes[2]. This may be an important reason of persistent infection of HBV. AICD in peripheral blood T-lymphocytes (PBL-Ts) of CHB in vivo has been approved by some reports, but does AICD occur more easily in PBL-Ts of CHB than in those of healthy control? In order to explore the role of AICD in chronic HBV infection, we studied and contrasted the difference of AICD in PBL-Ts from patients with CHB and from normal people in vitro.
Twenty-five patients (17 men, 8 women, aged 19-49, mean age 35.6 years) with CHB between March 2000 and April 2001 were selected from the Second Affiliated Hospital of Harbin Medical University. The diagnoses of all the patients were in accord with the Fifth National Conference on the Diagnostic Criteria of Virus Hepatitis (Beijing, 1995). And 14 healthy persons were selected as control.
10 mL peripheral blood was taken and heparin was added for anticoagulation. After equivalent Ficoll-Paque (from Amersham-Pharmacia, USA) was gently added, peripheral blood monocytes (PBMCs) were isolated by density gradient centrifugation (600 g, 20 min). Then PBL-Ts were purified with negative selection technique using immune-magnetic beads as follow. After PBMC was washed, mouse-anti-human anti-CD14, anti-CD16, anti-CD19 (2 μg·mL-1, DAKO Company, Denmark) were added and incubated for 30 min at 0 °C, centrifuged for removing the uncombined antibody. Then goat-anti-mouse CD3 mAb coating with magnetic beads (1 cell vs. 30 beads, Promega Company, USA) was added and incubated for 30 min at 0 °C. B cells, NK cells and monocytes were all linked with immune-magnetic beads and absorbed by magnetic stock (DAKO Company, Denmark). After the liquor was gently extracted by centrifugation, PBL-Ts were purified. The viability (95%) of the cells was confirmed by trypan blue staining. When detected by flow cytometry, the purity of PBL-Ts was over 97%.
After washed 3 times with PBS, 2 × 106·mL-1 PBL-Ts were added to a 24 well plate (NENC Company, USA) for cultivation. The samples were divided into treatment group and control group. The culture medium was RPMI1640 (GIBCO Company, USA) containing 10% calf blood serum (BANDIN TECH Company, China), penicillin (100 U·mL-1, BANDIN TECH Company, China) and streptomycin (100 U·mL-1, BANDIN TECH Company, China). The wells of treatment group were pre-coated with anti-CD3 mAb (5 μg·mL-1, DAKO Company, Denmark). Phorbol 12-myrisate 13-acetate (PMA) (50 ng·mL-1, Sigma Company, USA) and ionomycin (50 ng·mL-1, Sigma Company, USA) were added to the culture medium[3]. The culture medium of control group did not contain CD3, PMA and ionomycin. The liquid of culture medium was adjusted to 1 mL. After cultured for 14 h (37 °C, 5% CO2), PBL-Ts were harvested for AICD detection.
Some PBL-Ts were put on the carry sheet glass, dried naturally and fixed by 4% formaldehydum polymerisatum. Then, all the cells were stained with TdT-mediated dUTP nick end labeling (TUNEL, procedure according to clarification of the kit) (Promega Company, USA). The positive cells of TUNEL staining were detected by fluorescence microscope (BG-12, Olympass, Japan).
1 × 106 PBL-Ts were washed, fixed by1% formaldehydum polymerisatum, stayed overnight in 70% ethanol (-20 °C) and stained with TUNEL for apoptosis detection (procedure according to clarification of the kit). The apoptotic ratio of PBL-Ts was detected by a flow cytometer (Fort, B-D Company, USA).
100 μl supernatant of medium was collected respectively from each group after cultured for 14 h and frozen in -20 °C refrigerator for detection. The contents of IFN-γ and IL-10 were detected by using an ELISA kit. The parallel sample was set up for each sample. The OD450 value of each sample was measured with an enzyme label meter (550 model, Bio-RAD Program, USA), and then the content of each sample was converted according to the standard curve.
The data were presented as x-±s. ANOVA was used to compare the means.
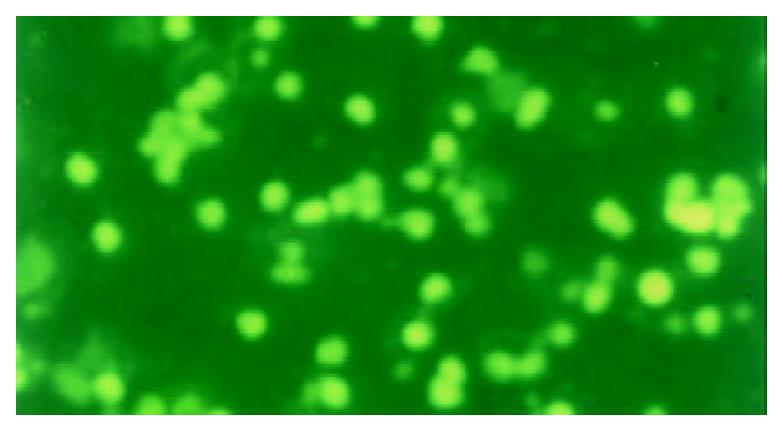
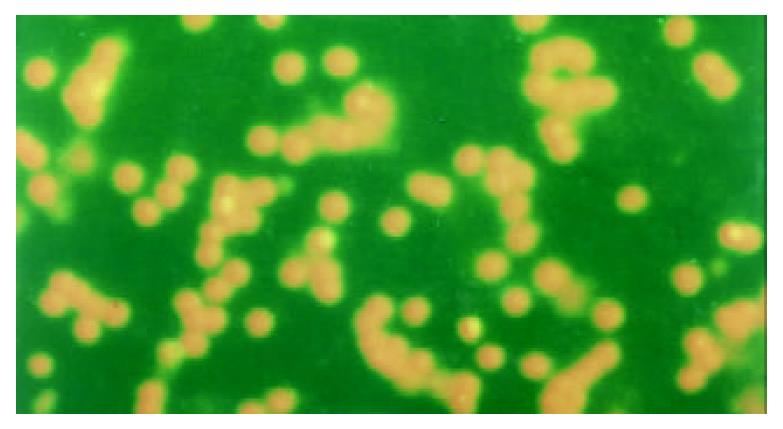
The apoptotic PBL-Ts presented DNA breakage. The breakage DNA could be linked by fluorescence labeling dUTP when TUNEL staining was adopted. The apoptotic cells took on kelly fluorescence under fluorescence microscope (Figure 1). This was named positive TUNEL staining. The plasm of PBL-Ts with AICD took on red fluorescence and the nuclei took on kelly fluorescence when TUNEL and PI double staining were adopted. But the cells without AICD only took on red fluorescence (Figure 2). The positive cells of TUNEL staining in PBL-Ts of CHB (with and without anti-CD3mAb, PMA and ionomycin) were more excessive than that of healthy control.
After cultivated for 14 h with induction, the PBL-Ts of CHB patients displayed distinct apoptosis. Apoptosis was also found in groups without anti-CD3 and other inductions, but their apoptotic ratio was lower. There was a similar AICD ratio of PBL-Ts between induction group and healthy control without induction. AICD ratio of PBL-Ts from CHB patients (with or without induction) was significantly higher than that from healthy control (Table 1).
Activated T lymphocytes may produce plentiful endogenous cytokine. Th1 mainly produces IFN-γ, IL-2 and TNF-α. But Th2 mainly produces IL-4, IL-5, IL-6, IL-10, etc. Cytokine IFN-γ, IL-10 were detected in this test. In all groups, the density of INF-γ in culture media of healthy control with induction group was the highest, and the patient in groups with induction was the lowest. But there was no difference among these groups in density of IL-10 (Table 2).
Chronic HBV infection is mainly related to the immune function of patients. In a large degree, immune tolerance, especially neonatal immune tolerance, results in persistence of chronic HBV infection. Because naive T cells are sensitive to Fas-mediated AICD and more easily deleted by Ag restimulation than primed T cells[4]. AICD of PBL-Ts plays a key role in central and peripheral immune tolerance[5,6]. AICD is one kind of apoptosis of reactivated lymphocytes when these lymphocytes are induced by activation signals (especially by complex of TCR/CD3). Ashwell and his colleagues first detected the AICD phenomenon in 1987 when they studied T lymphocyte hybrid tumors. AICD plays an important role in the negative selection of T lymphocytes in thymus, peripheral elimination and clearance of T lymphocytes that have already cleaned the foreign antigens. Therefore, AICD is an important mechanism in maintaining immunoregulation and achieving immune system homeostasis[6,7]. If one’s AICD mechanism is disordered (up-regulation or down-regulation), immune tolerance or autoimmune disease would occur.
In this experiment, AICD of PBL-Ts was successfully induced using anti-CD3 mAb, PMA and ionomycin. The responses of PBL-Ts from CHB and healthy control were different. The results indicated that when induced with anti-CD3, PMA and ionomycin in vitro, AICD ratio of PBL-Ts from CHB patients was significantly higher than that from healthy control and that from CHB patients without induction. But there was a similar AICD ratio of PBL-Ts between induction group and healthy control without induction. The results imply that AICD exists in PBL-Ts of CHB and causes decrease of T lymphocytes especially Th1 cells and functional defect. Specific immune response aiming directly at HBV should not occur. Finally, immunology tolerance to HBV would occur. Ji et al[8] using staphylococcus aureus enterotoxin B and rHBcAg proved that AICD of PBMCs in patients would lead to persistent infection of HBV.
Reduction of deferent cytokines in culture medium implies apoptosis of deferent subtype T lymphocytes, because the types of cytokine secreted by Th1 and Th2 are different. The detection results revealed that the density of INF-γ in culture media of induction groups from CHB was lower than that of other groups (P < 0.01). There was no difference between these groups in density of IL-10 (P > 0.05). These results imply AICD cells are mainly Th1 cells.
After infection of HBV, the virus elimination depends on specific cell immunity of the body. Mostly, specific cell immunity responses are induced by Th1 lymphocytes, but humoral immunity responses are induced by Th2 type lymphocytes. The sensitivity of the two types of T lymphocytes is not equal. The occurrence of AICD is easily induced by Th1 but not Th2 when induced by Anti-CD3 and corresponding antigen[9-11]. Fan et al[12-14] have proved that enhanced Th2 responses are present in chronic HCV infection, and this should be responsible for the persistent HCV infection. So, if specific PBL-Ts of CHB are reactivated by HBV antigens, AICD would occur mostly in Th1 type lymphocytes. Thus, specific cell immunity response aiming directly at HBV would be defective, and HBV permanent infection would occur. However, it would be a possible method to surmount immune tolerance and to clean HBV of CHB patients that we have managed to block the apoptosis of activated T lymphocytes[6,15] and raise the amount of specific T lymphocytes.
We are grateful to professor Hu-Lun Li (Harbin Medical University), Fang Liu, Wei Liu, Jin-Bai Guo, Shu-Yun Zhang, the staff members of Department of Infectious Diseases (the 2nd Affiliated Hospital, Harbin Medical University), Qin-Huan Wang (the First Affiliated Hospital, Beijing Medical University), Xue-Hai Zhang, Jin-Jian Bi (Jining Infectious Diseases Hospital) for their excellent technical assistant.
Edited by Wang XL
| 1. | Hartwig UF, Robbers M, Wickenhauser C, Huber C. Murine acute graft-versus-host disease can be prevented by depletion of alloreactive T lymphocytes using activation-induced cell death. Blood. 2002;99:3041-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Baumann S, Krueger A, Kirchhoff S, Krammer PH. Regulation of T cell apoptosis during the immune response. Curr Mol Med. 2002;2:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Kottilil S, Bowmer MI, Trahey J, Howley C, Gamberg J, Grant MD. Fas/FasL-independent activation-induced cell death of T lymphocytes from HIV-infected individuals occurs without DNA fragmentation. Cell Immunol. 2001;214:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Inaba M, Kurasawa K, Mamura M, Kumano K, Saito Y, Iwamoto I. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J Immunol. 1999;163:1315-1320. [PubMed] |
| 5. | Hamad AR, Schneck JP. Antigen-induced T cell death is regulated by CD4 expression. Int Rev Immunol. 2001;20:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Tanimoto Y, Kizaki H. Proteasome inhibitors block Ras/ERK signaling pathway resulting in the downregulation of Fas ligand expression during activation-induced cell death in T cells. J Biochem. 2002;131:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol Cell Biol. 2002;80:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Ji W, Wang HF, Feng CQ. Activation-induced cell death in peripheral blood mononuclear cells (PBMCs) from patients with chronic hepatitis B may be related to abnormal production of interleukin 12 and 10. J Viral Hepat. 2001;8:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Varadhachary AS, Peter ME, Perdow SN, Krammer PH, Salgame P. Selective up-regulation of phosphatidylinositol 3'-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J Immunol. 1999;163:4772-4779. [PubMed] |
| 10. | Gorak-Stolinska P, Truman JP, Kemeny DM, Noble A. Activation-induced cell death of human T-cell subsets is mediated by Fas and granzyme B but is independent of TNF-alpha. J Leukoc Biol. 2001;70:756-766. [PubMed] |
| 11. | Roozendaal R, Vellenga E, de Jong MA, Traanberg KF, Postma DS, de Monchy JG, Kauffman HF. Resistance of activated human Th2 cells to NO-induced apoptosis is mediated by gamma-glutamyltranspeptidase. Int Immunol. 2001;13:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Fan XG, Liu WE, Li CZ, Wang ZC, Lou LX, Tan DM, Hu GM. Determination of serum cytokines in individuals with HCV infection. Zhonghua Shiyan He Linchuangbing Duxue Zazhi. 2000;14:145-147. |
| 13. | Fan XG, Tang FQ, Yi H, Liu WE, Houghton M, Hu GL. Effect of IL-12 on T-cell immune responses in patients with chronic HCV infection. APMIS. 2000;108:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Fan XG, Liu WE, Li CZ, Wang ZC, Luo LX, Tan DM, Hu GL, Zhang Z. Circulating Th1 and Th2 cytokines in patients with hepatitis C virus infection. Mediators Inflamm. 1998;7:295-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | da Rocha Dias S, Rudd CE. CTLA-4 blockade of antigen-induced cell death. Blood. 2001;97:1134-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |