Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2325
Revised: July 25, 2003
Accepted: August 2, 2003
Published online: October 15, 2003
AIM: Adrenomedullin (ADM) is a potent vasodilator peptide. ADM and nitric oxide (NO) are produced in vascular endothelial cells. Increased ADM level has been linked to hyperdynamic circulation and arterial vasodilatation in cirrhotic portal hypertension (CPH). The role of ADM in non-cirrhotic portal hypertension (NCPH) is unknown. plasma ADM levels were studied in patients with NCPH, compensated and decompensated cirrhosis in order to determine its contribution to portal hypertension (PH) in these groups.
METHODS: There were 4 groups of subjects. Group 1 consisted of 27 patients (F/M: 12/15) with NCPH due to portal and/or splenic vein thrombosis (mean age: 41 ± 12 years), group 2 consisted of 14 patients (F/M: 6/8) with compensated (Child-Pugh A) cirrhosis (mean age: 46 ± 4), group 3 consisted of 16 patients (F/M: 6/10) with decompensated (Child-Pugh C) cirrhosis (mean age: 47 ± 12). Fourteen healthy subjects (F/M: 6/8) (mean age: 44 ± 8) were used as controls in Group 4. ADM level was measured by ELISA. NO was determined as nitrite/nitrate level by chemoluminescence.
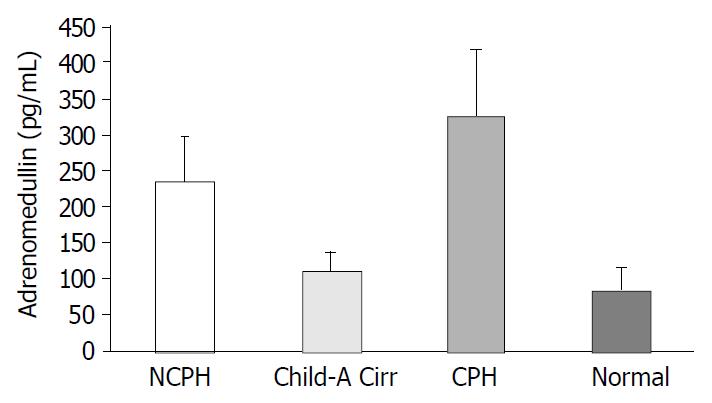
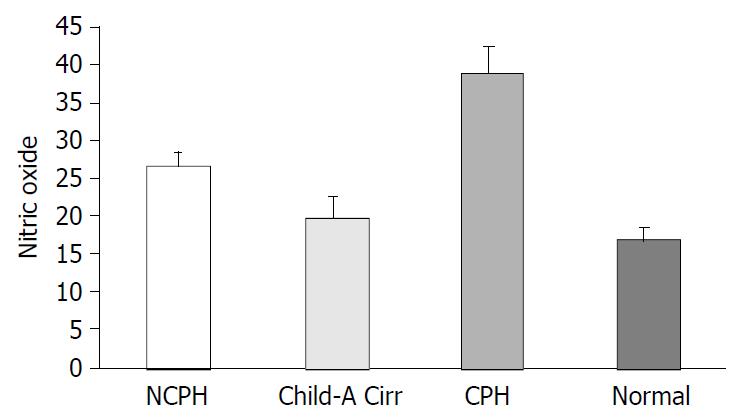
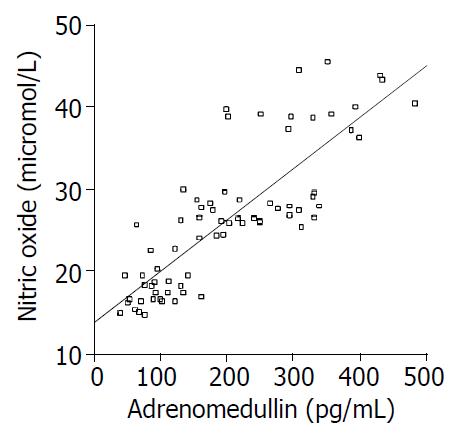
RESULTS: ADM level in Group 1 (236 ± 61.4 pg/mL) was significantly higher than that in group 2 (108.4 ± 28.3 pg/mL) and group 4 (84.1 ± 31.5 pg/mL) (both P < 0.0001) but was lower than that in Group3 (324 ± 93.7 pg/mL) (P = 0.002). NO level in group 1 (27 ± 1.4 μmol/L) was significantly higher than that in group 2 (19.8 ± 2.8 μmol/L) and group 4 (16.9 ± 1.6 μmol/L) but was lower than that in Group 3 (39 ± 3.6 μmol/L) (for all three P < 0.0001). A strong correlation was observed between ADM and NO levels (r = 0.827, P < 0.0001).
CONCLUSION: Adrenomedullin and NO levels were high in both non-cirrhotic and cirrhotic portal hypertension and were closely correlated, Adrenomedullin and NO levels increased proportionally with the severity of cirrhosis, and were significantly higher than those in patients with NCPH. Portal hypertension plays an important role in the increase of ADM and NO. Parenchymal damage in cirrhosis may contribute to the increase in these parameters.
- Citation: Tahan V, Avsar E, Karaca C, Uslu E, Eren F, Aydin S, Uzun H, Hamzaoglu H, Besisik F, Kalayci C, Okten A, Tozun N. Adrenomedullin in cirrhotic and non-cirrhotic portal hypertension. World J Gastroenterol 2003; 9(10): 2325-2327
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2325.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2325
Portal hypertension (PH) is a clinical condition characterized by specific hemodynamic abnormalities such as low arterial pressure, high cardiac output, over activity of vasoconstrictor systems and marked decrease in total systemic vascular resistance. Arterial vasodilatation and activation of several vasoactive and neurohumoral systems may play a key role in pathogenesis of sodium and water retention and ascites formation in cirrhosis[1,2]. These changes have been attributed to increased production of vasodilator substances[2,3]. Adrenomedullin (ADM) and nitric oxide (NO) are considered as essential mediators of hyperdynamic state. NO, a potent vasodilator substance synthesized from L-arginin by NO synthase, is increased in cirrhotic patients and experimental models of cirrhosis[3,4]. Specific NO inhibitors have been shown to counteract vasodilatation and hyperdynamic circulation in these groups[4,5]. In addition, circulating levels of potent vasodilating peptides (substance P, CGRP), pulsatile blood flow, and shear stress could contribute to the up regulation of endothelial NO-synthase (e-NOS)[3,6].
ADM is a potent endogenous vasorelaxing factor that was originally isolated from the extracts of human pheochromocytoma[7]. ADM is expressed in adrenal gland, lungs, kidneys, smooth muscle vascular endothelial cells, and splanchnic organs[3,8]. ADM level is increased after stimulation with bacterial endotoxin and cytokines[9]. The renal effects of ADM and part of its cardiovascular effects seem to be mediated by NO[10,11]. Previous studies reported that increased ADM level in cirrhotic patients occurred via increased production or decreased clearance of this substance, the elevation was more prominent in decompensated cirrhotic patients with marked PH[2,4,12,13]. Although ADM level is known to be increased in cirrhotic PH (CPH), there are no data concerning its level in non-cirrhotic PH (NCPH).
To our knowledge this is the first study determining plasma ADM concentrations in NCPH patients. We also aimed to compare the results within the group of compensated and decompensated cirrhotic patients (Child-Pugh A & C) and healthy subjects in order to estimate the contribution of cirrhosis and PH either separately or concomitantly to the elevation of ADM and NO level.
We compared ADM levels in the patients with compensated or decompensated cirrhosis and also in healthy subjects in order to determine whether portal hypertension or cirrhosis led to elevation in ADM and NO levels.
Our study included patients with NCPH, cirrhosis and healthy subjects as control. Cirrhosis was due to hepatitis C infection in all-cirrhotic cases, while in all cases of NCPH the origin was extrahepatic portal venous thrombosis. group 1 included 27 patients (F/M: 12/15 with NCPH due to portal and/or splenic vein thrombosis (mean age: 41 ± 12 years), Group 2 consisted of 14 (F/M: 6/8) compensated (Child-Pugh A) cirrhotic patients (mean age: 46 ± 4), group 3 included 16 (F/M: 6/10) decompensated cirrhotic (Child-Pugh C) patients (mean age: 47 ± 12), and group 4 consisted of 14 (F/M: 6/8) healthy subjects (mean age: 44 ± 8) taken as controls.
The diagnosis of PH and cirrhosis was established on the basis of clinical, biochemical and ultrasonographic findings and/or liver biopsy. Patients with active or recent gastrointestinal bleeding, bacterial infection, severe hepatic encephalopathy, cardiopulmonary disease and portal vein thrombosis within the previous ten days were excluded.
All patients and controls received restricted sodium diet (70 mmol/day) for at least 5 d before the study. Diuretics, beta-blockers and other cardioactive drugs were withheld during the 10-day period before the study. At 8.00 a.m. on the day of study, blood samples were drawn after the patients were fasted overnight and then waited for 45 min in supine position. This study was performed in accordance with the Declaration of Helsinki. A written informed consent was obtained from each patient and control subject participating in the study.
Nitric oxide heparinized whole blood was obtained by venipuncture, centrifuged (3000 rpm, 10 min, 0-40 C). Plasma concentration of NO was studied by chemoluminescence method using commercially available colorimetric assay (Roche, Cat No 1746081)[14].
Blood samples for ADM were collected into the vacutainer tubes, which contained EDTA. Blood was transferred from the vacutainer tubes to centrifuge tubes containing aprotinin (0.6 TIU/mL of blood) and gently rocked for several times to inhibit the activity of proteinases. Blood was centrifuged at 1600 G for 15 min at 4 °C. Plasma ADM concentration was measured by enzyme immunoassay (Phoenix Pharmaceuticals Inc. Harbor Boulevard, Belmont, California 94002) after extraction through the Sep-pak C-18 column supplied by the manufacturer[15,16].
Plasma in both parameters was immediately frozen and stored at -80 °C until assayed.
All results were expressed as mean ± standard deviation. Comparisons between the groups were performed by Kruskal-Wallis variance analysis and a P-value < 0.05 was accepted as statistically significant.
ADM level in Group 1 (236 ± 61.4 pg/mL) was significantly higher than that in Group 2 (108.4 ± 28.3 pg/mL) and Group 4 (84.1 ± 31.5 pg/mL) (both P < 0.0001) but was lower than that in Group 3 (324 ± 93.7 pg/mL)(P = 0.002) (Figure 1).
Nitrite/nitrate level in Group 1 (27 ± 1.4 μmol/L) was significantly higher than that in Group 2 (19.8 ± 2.8 μmol/L) and Group 4 (16.9 ± 1.6 μmol/L) but was lower than that in Group 3 (39 ± 3.6 μmol/L) (P < 0.0001 for all three) (Figure 2). A significant correlation was observed between ADM and NO levels (r = 0.827, P < 0.0001) (Figure 3).
Our study demonstrated increased plasma ADM and NO concentrations in both CPH and NCPH patients, the level of ADM was the highest in the CPH group. Increased ADM level was strongly associated with PH and plasma volume expansion[10,11]. In our series, ADM level was not different between compensated cirrhotic patients and controls but higher in both groups of patients with CPH and NCPH, evidencing the role of PH in the increase of ADM.
Cirrhotic patients showed hemodynamic abnormalities such as arterial hypotension, increased cardiac output and reduced systemic vascular resistance. These changes have been attributed to peripheral vasodilatation. The reduction in the effective blood volume, and the subsequent activation of renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system initiated renal sodium and water retention and ultimately led to ascites formation[11]. Furthermore, excessive production of endogenous vasodilatators played an important role in peripheral vasodilatation. ADM and NO are well known mediators in the pathophysiology of PH. ADM is a potent vasodilatator and natriuretic peptide[4]. ADM concentration was positively correlated with plasma renin activity and aldosterone, which are indicators of RAAS activity[1]. ADM had an indirect effect on hyperdynamic circulation. It might play a role in the mechanism of initiation of peripheral vasodilatation counterbalanced by the activation of RAAS and sympathetic nervous system and followed by renin and aldosterone increase. Previous studies reported that concentrations of ADM similar to those found in patients with ascites had vasodilatatory effect in the rat mesenteric circulation[2,12]. In addition, administration of ADM to anesthetized rat caused arterial hypotension, increased cardiac output and reduced systemic vascular resistance similar to the circulatory changes observed in cirrhosis[13]. All these changes may account for increased ADM level in patients with ascites and may also be seen in non-cirrhotic PH.
Cytokines were incriminated as to contribute to increased ADM levels via increased levels of TNF-α, interleukin-6 and bacterial endotoxin in advanced liver cirrhosis[18,19]. These factors are the result of parenchymal damage, which is known to stimulate the production of ADM by vascular smooth muscle and endothelial cells. However, a previous study reported that TNF-α and interleukin-6 levels showed very weak correlations with ADM levels[4]. In our study ADM level in CPH patients was significantly higher than that in NCPH patients. We conclude that cytokines are not completely responsible for the significant increase of ADM level in liver cirrhosis but they may contribute to this change.
Increased hepatic outflow resistance is the initial cause of CPH. It stimulates endothelial shear stress. Previous studies reported that ADM mRNA expression in endothelial cells was markedly increased by shear stress[20]. Thus ADM could be increased by the shear stress secondary to hyperdynamic circulation. ADM may also be produced in excess as an outcome of the volume expansion. Increased ADM level was found in cirrhotic chronic renal failure patients[2]. An increased ADM production to balance plasma volume overload might be an additional mechanism accounting for elevated ADM plasma concentration in patients with chronic renal disease.
NO is an important mediator of the hemodynamic alterations of liver cirrhosis. In our study ADM levels correlated with serum nitrite and nitrate levels. It was probably due to the fact that elevated NO levels in patients with advanced liver cirrhosis might further stimulate the production of NO and aggravate the vasodilatation in a vicious cycle[9,10].
In our study, ADM and NO levels in NCPH and advanced cirrhosis were higher than those in healthy controls and compensated cirrhotics. Likewise ADM and NO levels in CPH were higher than those in NCPH. Increased ADM level was closely correlated with NO level.
In conclusion, the results of our study suggest that portal hypertension per se is an independent factor for the elevation of ADM and NO in both cirrhotic and non-cirrhotic portal hypertension. Parenchymal destruction at various stages in cirrhosis may further contribute to the effects of these potent vasodilators and lead to a vicious cycle.
Edited by Wang XL
| 1. | Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 217] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Guevara M, Ginès P, Jiménez W, Sort P, Fernández-Esparrach G, Escorsell A, Bataller R, Bosch J, Arroyo V, Rivera F. Increased adrenomedullin levels in cirrhosis: relationship with hemodynamic abnormalities and vasoconstrictor systems. Gastroenterology. 1998;114:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Fernández-Rodriguez CM, Prada IR, Prieto J, Montuenga LM, Elssasser T, Quiroga J, Moreiras M, Andrade A, Cuttitta F. Circulating adrenomedullin in cirrhosis: relationship to hyperdynamic circulation. J Hepatol. 1998;29:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Genesca J, Gonzalez A, Catalan R, Segura R, Martinez M, Esteban R, Groszmann RJ, Guardia J. Adrenomedullin, a vasodilator peptide implicated in hemodynamic alterations of liver cirrhosis: relationship to nitric oxide. Dig Dis Sci. 1999;44:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Pizcueta P, Piqué JM, Fernández M, Bosch J, Rodés J, Whittle BJ, Moncada S. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology. 1992;103:1909-1915. [PubMed] |
| 6. | Fernandez-Rodriguez CM, Prieto J, Quiroga J, Zozoya JM, Andrade A, Nunez M, Sangro B, Penas J. Plasma levels of sub-stance P in liver cirrhosis: relationship to the activation of vaso-pressor systems and urinary sodium excretion. Hepatology. 1995;21:35-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1585] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 8. | Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett. 1994;338:6-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 402] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Interleukin-1, tumor necrosis factor and lipopolysaccharide additively stimulate production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;207:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 243] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Ishimitsu T, Nishikimi T, Saito Y, Kitamura K, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994;94:2158-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 341] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Jougasaki M, Rodeheffer RJ, Redfield MM, Yamamoto K, Wei CM, McKinley LJ, Burnett JC. Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest. 1996;97:2370-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Fábrega E, Casafont F, Crespo J, de la Peña J, San Miguel G, de las Heras G, García-Unzueta MT, Amado JA, Pons-Romero F. Plasma adrenomedullin levels in patients with hepatic cirrhosis. Am J Gastroenterol. 1997;92:1901-1904. [PubMed] |
| 13. | Kojima H, Tsujimoto T, Uemura M, Takaya A, Okamoto S, Ueda S, Nishio K, Miyamoto S, Kubo A, Minamino N. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J Hepatol. 1998;28:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8653] [Cited by in RCA: 9032] [Article Influence: 210.0] [Reference Citation Analysis (0)] |
| 15. | Porstmann T, Kiessig ST. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150:5-21. [PubMed] |
| 16. | Avrameas S. Amplification systems in immunoenzymatic techniques. J Immunol Methods. 1992;150:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 18. | Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 290] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 331] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Chun TH, Itoh H, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Doi K, Inoue M, Masatsugu K. Shear stress augments expression of C-type natriuretic peptide and adrenomedullin. Hypertension. 1997;29:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |