INTRODUCTION
Research on using plants for expression and delivery of oral vaccine has attracted much academic attention and has become a hot spot of study since 1990 when Curtiss et al first reported the expression of Streptococcus mutans surface protein antigen A (SpaA) in tobacco, and great progress has been made since then[1]. So far, more than 10 viral epitopes and subunits of bacterial toxins have been successfully expressed in plants, mainly including hepatitis B surface antigen (HBsAg)[2-9], E.coli heat-labile enterotoxin B subunit (LT-B)[10-15], cholera toxin B subunit (CT-B)[16], Norwalk virus capsid protein (NVCP)[17,18], rabies virus glycoprotein[19], etc. The involved plant receptors are mostly mode species: tobacco, potato, etc., some fruits and vegetables that can be consumed raw, tomato, banana, lettuce and lupine have also been attempted. Significant breakthrough has been achieved in increasing the expression of vaccine components in plants. At present, the plant-derived oral vaccines from potato for HBsAg and LT-B are undergoing clinical trials[13,14], but there has been no report about plant-derived oral vaccine for hepatitis E virus (HEV).
HEV is the major cause of acute, enteric non-A, non-B (NANB) hepatitis in the world, and large outbreaks occur primarily in underdeveloped countries. China is one of the high epidemic areas. The highest infection rate is in young adults (15-40 years). Although only 1% to 3% of non-pregnant patients with HEV infection progress to fatal fulminant hepatitis, the mortality can be as high as 20% in pregnant patients[20]. There is neither effective antiviral drugs nor vaccine against HEV available for commercial use at present. Since HEV pathogen is difficult to culture, it is not easy to develop the live attenuated strains for vaccine. A promising approach is to develop recombinant subunit vaccines. Compared with gene engineering vaccine for hepatitis B, the study of hepatitis E recombinant vaccine was only a recent endeavor, but some progress has been made.
ORF2-encoded protein of HEV is the most promising subunit vaccine candidate because it possesses good antigenicity. So far, HEV ORF2 gene or its fragments have been expressed in prokaryote cells[21-24], insect cells[25], animal cells[26], and Pichia pastoris[27], etc., and the expression products possessed immunogenicity. HEV vaccine is about to undergo clinical trials[28].
Since hepatitis E occurs primarily in developing countries and impoverished regions with poor environmental sanitation, where administration of various medicinal vaccines may be hampered by the relatively high cost. Plant-derived hepatitis E vaccine is a promising approach that may solve the problem because of its low cost for delivery and administration, and its safety for humans.
Tomato is a nutrition-rich fruit that can be consumed raw and easily transformed, so it is an ideal plant carrier for oral vaccine. In this paper we reported that a plant expression vector of HEV antigen gene was constructed and transformed into tomato plants with Agrobacterium tumefaciens, and its expression in plants and immunoactivity of the expression product were examined. This study would lay the foundation for further research on the development of a new type of plant-derived hepatitis E oral vaccine or other oral vaccines, and promote their practical application.
MATERIALS AND METHODS
Plant material
Tomato (Lycopersium esculentum CV. “XiuNu”) seeds were purchased from Xiamen Nong-You Seed Co., Ltd.
Reagents, bacteria and plasmids
Restriction endonucleases and T4 DNA ligase were obtained from Promega Co. Hygromycin and X-gluc staining solution from Calbiochem-novabiochem Co. and Amres Co., respectively. Double antibody sandwich-ELISA kit was provided by Beijing Wantai Biological Pharmaceutical Co. Agrobacterium tumefaciens strain EHA105 was kindly presented by Professor Zhang Qi-fa, Huazhong Agricultural University. Plasmid pBPFΩ7 containing CaMV35s promoter and nos terminator, and plant binary plasmid pCAMBIA1301 containing hygromycin-resistant gene, kanamycin-resistant gene and Gus gene, were constructed and preserved in our laboratory.
Construction of plant binary expression vector
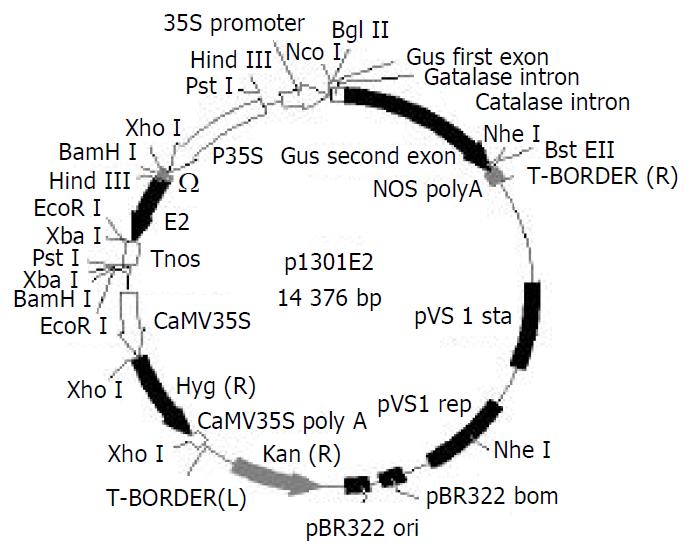
An 810 bp DNA fragment (named E2) of HEV ORF2 region, located between amino acid residue 394 and 604[23], was obtained by a PCR-based assembly from the patient’s serum and inserted into pBPFΩ7 between CaMV35S promoter and nos terminator at BamHI/EcoRI site to form pBE2. The fragment containing “P35S + Ω + E2 + Tnos” was isolated by gel extraction from plasmid pBE2 after PstI restrictive digestion and then subcloned into plasmid pCAMBIA1301 that had been digested by the same restriction endonuclease to yield the reconstructed plant binary expression plasmid p1301E2 (Figure 1). Confirmed by restriction digestion, p1301E2 was directly introduced into Agrobacterium tumefaciens strain EHA105 by freeze-thaw method.
Figure 1 Structure of plasmid p1301E2.
Plant transformation and regeneration
Tomato was transformed through leaf discs mediated by Agrobacterium tumefaciens EHA105 with p1301E2. Shoots were generated from transformed callus after 3-4 weeks selected on medium containing 20 mg of hygromycin (Hyg) and 300 mg of cefotaxime per liter. The rooting was obtained in medium containing 20 mg of Hyg per liter, and the plantlets was transplanted to soil, and watered with 1/2 MS medium.
Analysis of Gus gene expression
Both transformed and untransformed tissues were cut from tomato plants, immerged into Gus reaction buffer (X-gluc staining solution) for 12 to 24 hours at 37 °C, then bleached with absolute alcohol, observed and photographed under dissecting microscope.
Analysis of HEV-E2 gene integration
PCR amplification Genomic DNAs extracted from leaves of tomato plants by CTAB[29] were used as PCR templates. The forward primer HEFP and reverse primer HERP were: 5’-GGATCCATATGCAGCTGTTCTACTCTCGTC-3’ and 5’-CTCGAGAAATAAACTATAACTCCCGA-3’, respectively (synthesized by BioAsia Co., Shanghai). PCR reaction was performed using 50 ng of template DNA, 0.5 μM of each primer in a total volume of 30 μL. Cycling parameters were at 94 °C for 10 min, followed by 35 cycles at 94 °C for 50 s, at 57 °C for 50 s, and at 72 °C for 50 s, and a final extension at 72 °C for 7 min.
Southern dot blotting It was performed as reported previously[29].
Analysis of HEV-E2 gene expression
ELISA Total soluble proteins were extracted from leaf and fruit tissues as described[29], and HEV-E2 recombinant protein was detected by HEV enzyme-linked immunosorbant assay (ELISA) kit, the protocol and positive determination were performed according to the instructions supplied with the kit. The expression levels of HEV-E2 in transformants were quantified by ELISA. The extract of transformant was diluted several fold until it could reach the same OD value (measurement wavelength: 450 nm) as the standard HEV-E2 protein (1 ng/mg), then HEV-E2 expression levels in transformants could be calculated according to the sampling quantity, and diluting times.
RESULTS
Regeneration of transgenic tomato plants
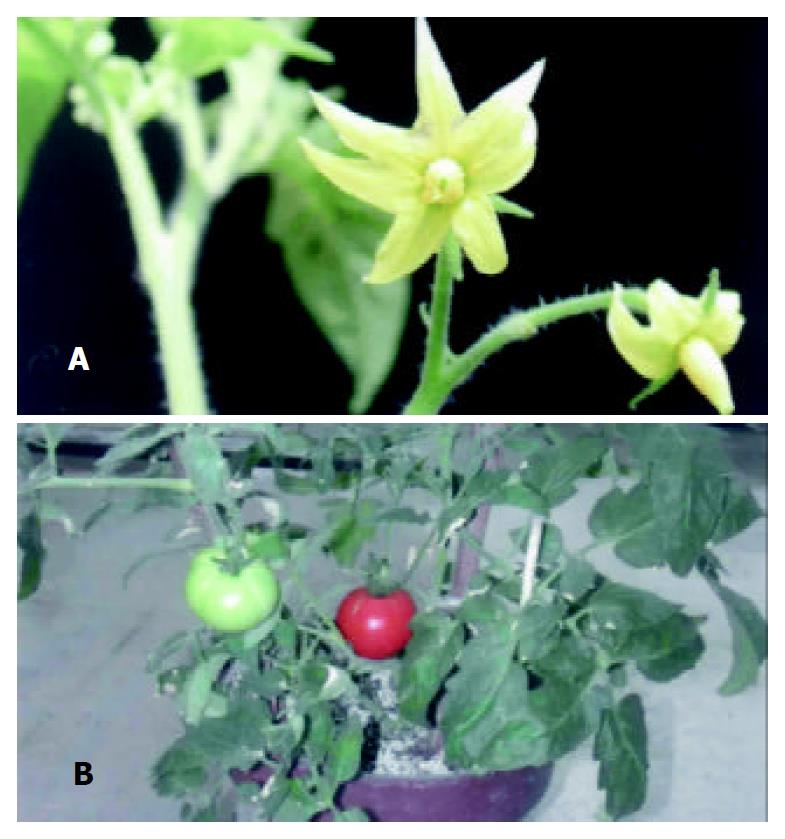
The plasmids for expression of HEV-E2 in plants allowed morphogenesis of transformants on selective medium containing hygromycin. Through about 1 month of selection, 7 independent Hyg-resistant plantlets were obtained. These transformants bore fruits and the seeds were collected. The flowers and fruits of the transformants resembled those of wild-type tomato plants and the seeds were plump. Each fruit produced approximately 70 seeds. This demonstrated that the introduction of foreign gene into tomato plants did not influence the normal growth and development of the transformants (Figure 2).
Figure 2 Transgenic plants in greenhouse.
A: Flowers of transformants, B: Fruits of transformants.
Gus expression in transgenic plants
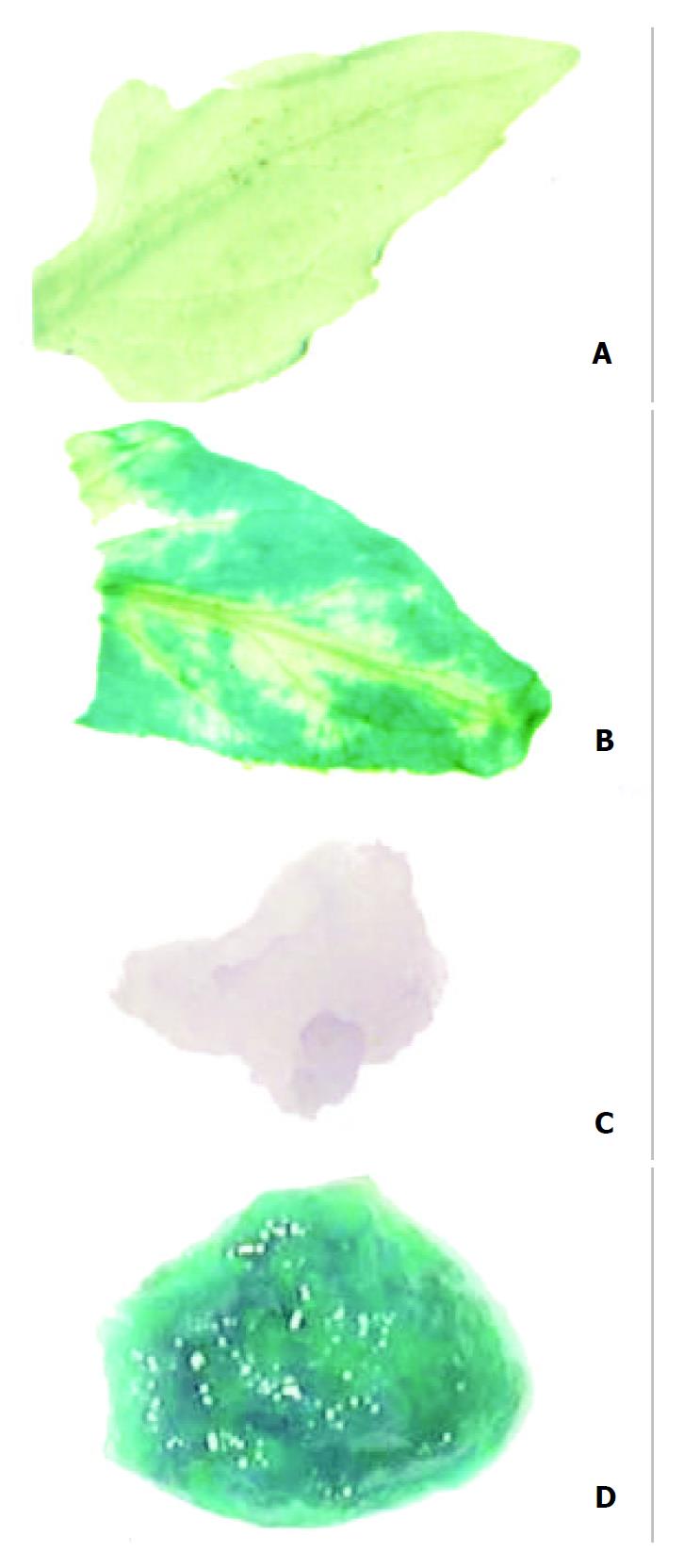
Untransformed tissues appeared colourless when stained with Gus reaction solution and bleached with absolute alcohol, whereas transformed tissues presented blue spots even after bleached with absolute alcohol, the spots were generally large in size, with a deep blue color (Figure 3). The results indicated that Gus gene was stably integrated into the genomic DNA of transformed tomatoes.
Figure 3 Analysis of Gus gene expression.
A: Leaf of untransformed tomato plant, B: Leaf of transformed tomato plant, C: Flesh of untransformed tomato, D: Flesh of trans-formed tomato.
PCR amplification
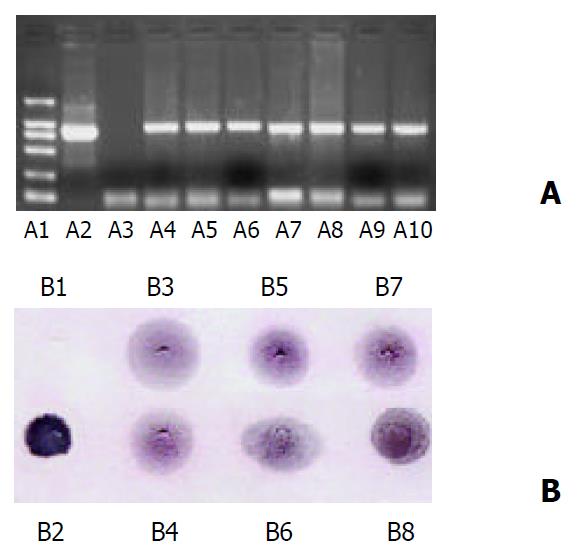
The expected 810 bp fragments were amplified from 7 transformed lines of tomatoes, the length of the fragments was identical with that amplified from p1301E2 (positive control), whereas there was no PCR product in wild-type tomato plants (negative control). The results primarily verified that the target gene was integrated into the genomic DNA of transformed tomatoes (Figure 4A).
Figure 4 Analysis of HEV-E2 gene integration.
A: PCR amplifi-cation of genomic DNA of tomato plants, B: Southern dot blot-ting of genomic DNA of tomato plants. A1: DL-2000 marker, A2: Positive control (p1301E2), A3: Total DNA of wild-type con-trol plant, A4-A10: Total DNA of independent transformants. B1: Total DNA of wild-type control plant, B2: Positive control (p1301E2), B3-B8: Total DNA of independent transformants.
Southern dot blotting
To further verify the integration of foreign gene into tomato plants, the total genomic DNA of transformed tomatoes was hybridized by a DIG-labeled probe (encompassing the coding region of HEV-E2 gene) generated by PCR amplification from p1301E2 with HEFP and HERP primers, and all the transformants gave the same hybridization spots as the positive control (p1301E2) did, whereas the untransformed plant showed no detectable hybridization signal (Figure 4B, Figure 5). Thus the results further confirmed that the target gene was integrated into the tomato plants.
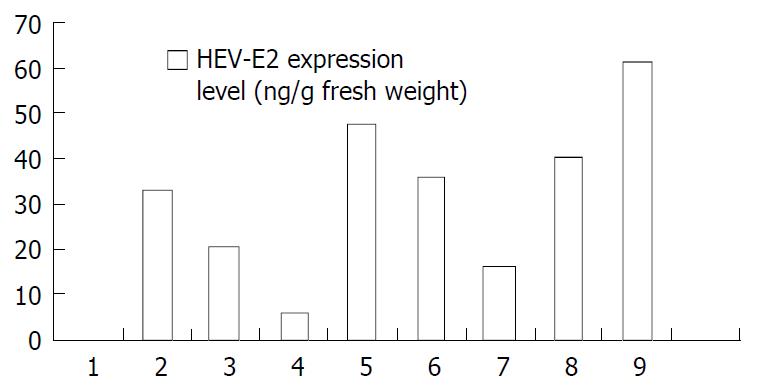
Figure 5 HEV-E2 expression levels in transgenic tomato plants.
1: Leaves of wild-type control plant, 2-8: Leaves of seven inde-pendent transformants, 9: The mixed flesh tissue of 3 indepen-dent transformant fruits.
Expression of HEV-E2 gene in transgenic tomato plants
The leaf extracts of 7 lines of transformants and the mixed fruit tissue extracts from 3 transformants were tested by ELISA for the presence of HEV-E2 expression products, and foreign protein could be detected in all of the examined samples. The reaction was specific because wild-type tomato showed no detectable expression products. The result demonstrated that HEV-E2 gene was expressed in transformed tissue. The expression level of HEV-E2 protein was lower as compared to HBsAg in transgenic tomatoes[8], the maximal expression level was only 47.9 ng/g fresh weight in leaves. The expression levels were different between different transformants and between different organs of the same plant, which indicated that the inserting site of foreign DNA into the plant genome was random. The expression in fruits (61.22 ng/g fresh weight) was higher than that in leaves, which was similar to that of HBsAg in transgenic tomatoes[8]. The higher expression of target protein in edible tissue mightbe helpful for producing oral vaccine.
DISCUSSION
The most striking advantage of using tomato as oral vaccine vector is that the plant-derived vaccine is cheap and tomato is a freshly-eaten fruit, which allows the target population to acquire immunity at the same time when they enjoy the delicious fruits. But accumulation of protein in tomato itself is low, the expression levels of foreign protein in it are much lower. This will make it difficult when administering the plant-derived vaccine. However, the expression of foreign protein in plants might be increased by several modifications, including the use of stronger promoters, the use of plant-derived leader sequences and signal peptide, and targeting the protein for retention in edible tissue and so on[1]. For example, Mason et al[2] increased the level of HBsAg 11 fold in transgenic tobacco by linking CaMV35S promoter to the tobacco etch virus (TEV) 5’ leader sequence (acts as a translational enhancer). In 1998, Mason et al[12] increased the expression of LT-B in transgenic potatoes 3-14 times by designing and constructing a plant-optimized synthetic gene encoding LT-B. Tackaberry et al[30] reported the synthesis of recombinant glycoprotein B which expressed specifically in tobacco seeds, with expression level reaching 70-146 ng/mg extracted protein. Lauterslager et al[11] made a synthetic gene coding for LT-B and optimized it for expression specifically in potato tubers and accumulation in endoplasmic reticulum, which resulted in a high expression level about 13 µg/g fresh weight of LT-B in potato tubers. Besides, by using chloroplast expression system, Cosa et al[31] introduced foreign genes into the chromoplast genome, which enabled the foreign protein to accumulate at 46% of the total soluble protein in leaves of transgenic plants. All these studies provided us successful experiences in improving expression level of HEV-E2 in transgenic tomatoes. In our study, we only inserted an enhancer Ω in the promoter, there were many reconstruction possibilities to achieve high expression. Currently we are engaged in researches on increasing expression level of foreign proteins, and the animal trials of oral immunization of mice with transgenic tomatoes expressing HEV-E2 are also in progress.
We have successfully introduced HEV-E2 gene into tomatoes and identified the expression protein. The expression product possessed HEV specific antigenicity in transgenic plants. Previous studies also demonstrated that plant-derived vaccines were safe and functional. It is cheap to produce and store, easy to deliver and administer, it has many advantages over other vaccines. Although this technology is not likely to produce great commercial value in the short term, the present advance in plant genetic engineering demonstrates that there is a tremendous potential to develop various low-cost recombinant vaccines by using plants.