Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2182
Revised: May 25, 2003
Accepted: June 2, 2003
Published online: October 15, 2003
AIM: To study the immunoprotective effect of liver cancer vaccine with co-transfected IL-2 and B7-1 genes on hepatocarcinogenesis in mice.
METHODS: The murine liver cancer cell line Hepal-6 was transfected with IL-2 and/or B7-1 gene via recombinant adenoviral vectors and the liver cancer vaccines were prepared. C57BL/6 mice were immunized with these vaccines and challenged with the parental Hepal-6 cells afterwards. The immunoprotection was investigated and the reactive T cell line was assayed.
RESULTS: The immunoprotection of the tumor vaccine was demonstrated. The effect of IL-2 and B7-1 genes co-transfected Hepal-6 liver cancer vaccine (Hep6-IL2/B7 vaccine) on the onset of tumor formation was the strongest. When attacked with wild Hepal-6 cells, the median survival period of the mice immunized with Hep6-IL2/B7 vaccine was the longest (68 d, χ2 = 7.70-11.69, P < 0.05) and the implanted tumor was the smallest (z = 3.20-44.10, P < 0.05). The effect of single IL-2 or B7-1 gene-transfected vaccine was next to the IL2/B7 gene co-transfected group, and the mean survival periods were 59 and 54 d, respectively. The mean survival periods of wild or enhanced green fluorescence protein gene modified vaccine immunized group were 51 and 48 d, respectively. The mice in control group all died within 38 d and the implanted tumor was the largest (z = 3.20-40.21, P < 0.05). The cellular immunofunction test and cytotoxicity study showed that the natural killer (NK) cell, lymphokine activated killer (LAK) cell and cytotoxic T lymphocyte (CTL) activities were significantly increased in mice immunized with the Hep6-IL2/B7 vaccine, (29.5% ± 2.5%, 65.0% ± 2.9%, 83.1% ± 1.5% respectively, compared with other groups, P < 0.05).
CONCLUSION: The Hep6-IL2/B7 liver cancer vaccines can induce the mice to produce activated and specific CTL against the parental tumor cells, and demonstrate stronger effect on the hepatocarcinogenesis than single gene modified or the regular tumor vaccine. Therefore, the vaccines may become a novel potential therapy for recurrence and metastasis of HCC.
- Citation: Ge NL, Ye SL, Zheng N, Sun RX, Liu YK, Tang ZY. Prevention of hepatocellular carcinoma in mice by IL-2 and B7-1 genes co-transfected liver cancer cell vaccines. World J Gastroenterol 2003; 9(10): 2182-2185
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2182
Hepatocellular carcinoma (HCC) is the most common primary malignancy of liver in humans and the rate of incidence and mortality is very high in East Asia and China[1]. Though many approaches, such as surgical resection, transarterial chemoembolization (TACE), percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), radiotherapy and liver transplantation were developed to treat it, and the effective and survival rates were increased, a large number of patients died from recurrence and metastasis[2-6].
Some studies showed immunogene modified tumor vaccines could induce effective specific active T cell immune response to prevent tumor recurrence and metastasis[7-10]. Also some reports demonstrated that specific active immunotherapy based on specific antitumor T cell immunity could be a potential modality for further improving the survival of HCC patients on preventing tumor recurrence and metastasis[11-14]. But HCC is poorly immunogenic. Poor or no immunogenicity and lack of costimulating molecules on the surface of tumor cells are some of the causes of most tumors including HCC[15].
The successful induction of an anti-tumor immune response has been reported in a number of tumor models including HCC animal models by using B7-1 (CD80) transfected tumor cells as a vaccine[16]. The rationale of B7-1 based immunotherapy is that T cells require both an antigen-specific signal delivered through the T-cell receptor and a co-stimulatory signal to be fully activated and proliferated, and secrete cytokines or generate cytotoxic T lymphocyte (CTL) to cytolyse tumor cells[17-19].
Interleukin 2 (IL-2) is a growth factor that stimulates the proliferation of cytotoxic T cells, helper T cells, natural killer (NK) cells, and lymphokine activated killer (LAK) cells, all of which can participate in the anti-tumor response[20-22]. Many HCC animal models demonstrated that local secretion of IL-2 abrogated the tumorigenicity of cytokine-producing tumor cells and inducing a long-lasting protective immune response against a subsequent tumor graft[23,24].
In this study, we compared the effect and immunological mechanism of mouse Hepal-6 liver cancer cell vaccine modified by IL-2 and/or B7-1 genes on protecting the C57BL/6 mice from challenge of wild parental Hepal-6 cells. The results provide some experimental evidences that gene modified tumor vaccine can prevent recurrence and metastasis of liver cancer.
For the experiments, three tumor cell lines were used. Hepal-6 was a liver cancer cell line derived from C57BL/6 mice generously provided by Dr. Li-Xin Wei from Eastern Hepato-Biliary Surgery Hospital, Second Military Medical University, Shanghai. P815 (NK cell resistant) and Yac-1 (NK cell sensitive) were leukemia cell lines derived from C57BL/6 mice and generous gifts from Prof. Xue-Tao Cao from Institute of Immunology, Second Military Medical University, Shanghai.
Female C57BL/6 mice, 6-8 weeks old (15-19 g), were purchased from the Animal Center of Chinese Academy of Sciences and housed in a specific-pathogen-free animal facility in Shanghai Medical University.
Recombinant adenoviruses carrying human IL-2 and B7-1 genes (AdVhIL-2 and AdVhB7-1) were obtained from Prof. Xue-Tao Cao. The control AdVEGFP (recombinant adenovirus carrying enhanced green fluorescence protein) was gifted from Dr. Gambotto from Pittsburgh University, USA.
CD3-FITC, CD4-PE, CD8-PE and CD25-PE, monoclonal antibodies (mAbs) specific for murine CD3, CD4, CD8 and CD25 respectively,used for flow cytometry were purchased from Pharmingen Co. (USA). MTS kits used for cytolytic assay were purchased from Promega Co. (USA). CD80-FITC which was mAbs specific for human CD80 was purchased from Pharmingen Co. (USA). Human IL-2 ELISA Kits used to detect the active part p70 of IL-2 in solution were purchased from DIACLONE Co. (USA).
Hepal-6 cells were transfected with various recombinant adenoviruses according to the five protocols listed below, namely 200 pfu/cell AdhIL-2, 200 pfu/cell AdhB7-1, co-transfection of 200 pfu/cell AdhIL-2 and 200 pfu/cell AdhB7-1, 200 pfu/cell AdEGFP, and wild Hepal-6 cell not transfected with any gene. All of the modified Hepal-6 cells in the five groups were inactivated with mitomycin-C (80 μg/mL) to prepare tumor vaccines. These tumor vaccines were named as Hep6-IL2, Hep6-B7, Hep6-IL2B7, Hep6-EGFP and Hep6 tumor cell vaccines, respectively.
The mice were divided into six groups with 6 mice in each. Five groups were injected 5 × 106 cell vaccines/mouse subcutaneously in the left scapula of those above 5 tumor cell vaccines, respectively. The sixth group was control group and the mice were injected culture medium. All the mice were injected twice a week.
On the 7th day after the final immunization, the mice were injected 8 × 106/mouse of wild Hepal-6 cell subcutanously in right scapula. The mice were scored for tumor growth twice a week according to the tumor volume, and calculated as V = L × W2/2 (L: length, W: width)[25]. The survival time of the mice was investigated.
The mice in each group were killed by dislocating cervical vertebra on the 7th day after the final immunization, and the spleens were cut aseptically and minced into suspension of single splenocytes. Then the suspensions were centrifuged at 1000 rpm for 5 min and RBCs in the splenocytes were lysed with sterile distilled water for 10 sec to get lymphocytes, and then the debris was filtered. The filtered cells were centrifuged, and the lymphocytes were harvested and maintained in 1640 medium (GIBCO BRL, USA) containing 10% FCS (HYCLONE, USA).
The freshly prepared spleocytes were analyzed by direct immunofluorescence staining with mAbs CD3-FITC, CD4-PE, CD8-PE and CD25-PE. Flow cytometry analysis was then performed with a FACScan (Becton Dickson Co.) in the Department of FACS, the Chinese Academy of Sciences.
The harvested lymphocytes (2 × 106 cell/mL) were cultured in 1640 containing 10% FCS with rhIL-2 at 1000 U/mL at 37 °C in a 5% CO2 incubator for 5 d and then cocultured with mitomycin Ctreated Hepal-6 cells (20:1 of responder to tumor cell ratio ) in 1640 containing 10% FCS with rhIL-2 at 50 U/mL at 37 °C in a 5% CO2 incubator for 5-7 d.
MTS assay was employed to test the cytolytic activity of NK cell, LAK cell and CTL from stimulated lymphocytes. Target cells were Yac-1, P815 and Hepal-6 respectively and incubated with various lymphocytes at 37 °C in a 5% CO2 incubator for 2-4 hours at 100:1 of effector/target ratio. Assays were performed in triplicate wells. Cytolytic activity was calculated according to the formula provided by the MTS kits: cytolytic activity (%) = [(ODe-ODb) + (ODt-ODb)-(ODa-ODb)] × 100%/(ODt-ODb).
The data were analyzed with SAS 6.12 and STATA 6.0 software, and expressed as x-±s. The cytolytic activity of NK cell, LAK cell and CTL was determined by t test. The growth curves of tumor volume were analyzed with a generalized regression model and the mice survival period was analyzed with Log-Rank test.
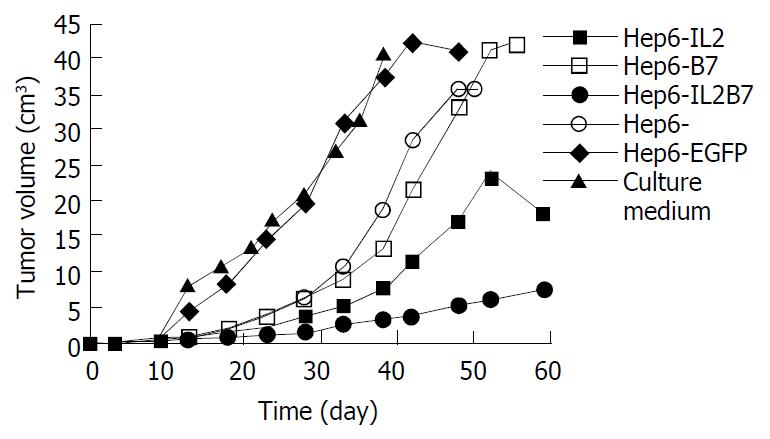
After the immunized mice were challenged with wild Hepal-6 cells, the change of the tumor volume was assessed as is shown in Figure 1. The tumors grew progressively in Hep6-EGFP and Hep6 tumor vaccine immunized mice. The tumors in Hep6-IL2 and Hep6-B7 tumor vaccine immunized mice grew more slowly than those in Hep6-EGFP, Hep6 tumor vaccine immunized mice and the control. Compared with the above 5 groups of mice, the tumors in Hep6-IL2B7 tumor vaccine immunized mice grew most slowly and the tumor volume was the smallest (z = 3.20-44.10, P < 0.05) and about 50% of the tumor disappeared 68 d after attacked by the wild Hepal-6 cell.
The survival periods of each group of mice were compared. The median survival time of Hep6, Hep6-EGFP, Hep6-B7 and Hep6-IL2 tumor vaccine immunized mice was 51, 48, 54 and 59 d, respectively and significantly longer than that of the control group (χ2 = 10.61-23.81, P < 0.05). The median survival period of the control mice was the shortest and all the mice died within 41 d. The median survival period of the Hep6-IL2B7 tumor vaccine immunized mice was significantly longer than that of other groups (χ2 = 7.70-11.69, P < 0.05) and all the mice in this group were alive for more than 68 d after attacked by the wild Hepal-6 cell.
Flow cytometry analysis confirmed that the transfected tumor vaccines Hep6-IL2B7 and Hep6-B7 expressed a higher level of CD80 (55.47% and 59.11%, respectively) than Hep6-IL2, Hep6 and Hep6-EGFP tumor vaccines (12.48%, 11.87%, and 7.93%, respectively).
In the supernant of Hep6-B7, Hep6 and Hep6-EGFP tumor vaccines, no IL-2 was detected, while a high level IL-2 was detected in the supernant of Hep6-IL2 and Hep6-IL2B7 tumor vaccines (1885 pg and 460 pg per 3 × 105 cells, respectively).
The CD4/CD8 ratio in lymphocytes of the Hep6-IL2B7 tumor vaccine immunized mice was the lowest (0.91) among all the study groups and the CD25 positive rate was the highest (32.85%). The CD4/CD8 ratios in lymphocytes of the Hep6-B7, Hep6-IL2 and Hep6 tumor vaccine immunized mice and normal mice were 1.02, 1.17, 1.24 and 1.31, respectively, while the CD25 positive rates were 28.62%, 26.13%, 19.32% and 3.59%, respectively.
The results are shown in Table 1. The cytolytic activities of the NK cells in each group of tumor vaccine immunized mice were all significantly higher than those in the normal mice (t = 11.3-15.5, P < 0.05) and it was the highest in the Hep6-IL2B7 group (29.5% ± 2.5%, t = 10.4-15.5, P < 0.05). The cytolytic activities of the LAK cells in Hep6-IL2 and Hep6-IL2B7 tumor vaccine immunized mice were significantly higher than those in the normal mice (60.9% ± 1.7%, 65.0% ± 2.9%, t = 10.6-40.2, P < 0.05). The cytolytic activities of the CTL induced by Hep6-IL2B7 tumor vaccine were significantly higher than those in other groups (83.1% ± 1.5%, t = 13.7-53.3, P < 0.05).
| Effector cell | Target cell | ||
| Yac-1 | p815 | Hepal-6 | |
| Normal group | 19.0 ± 1.9 | 48.4 ± 1.9c | 29.9 ± 1.3e |
| Hep6-IL2 cancer vaccine | 32.5 ± 2.7a | 60.9 ± 1.7 | 64.4 ± 12.3e |
| immunized group | |||
| Hep6-B7 cancer vaccine | 17.0 ± 1.7 | 47.7 ± 1.2c | 71.2 ± 1.5e |
| immunized group | |||
| Hep6-IL2B7 cancer vaccine | 29.5 ± 2.5a | 65.0 ± 2.9 | 83.1 ± 1.5 |
| immunized group | |||
| Hep6 cancer vaccine | 18.5 ± 1.4 | 42.5 ± 1.3c | 52.8 ± 1.2e |
| immunized group | |||
Hepatocellular carcinoma was poor immunogenicity partly because of poor antigen expression and lack of co-stimulatory molecules[26]. Though successful induction of anti-tumor immunity by means of B7-1 gene[11,14,16] or cytokine (IL-2, IL-4, IL-12, GM-CSF, IFN, and so on) gene[9,12-14,27] transfection has been reported in some HCC animal models, prevention of tumor recurrence and metastasis still needs to be improved.
Some animal experiments showed that IL-2 gene-modified tumor vaccine constantly secreted a relatively high level of IL-2 in local areas, induced local and systemical specific antitumor immuno-reactions and immuno-memory T cells in the body of the animals, prevented the challenge of the next parental tumor and decreased the recurrence rate[28-30]. Reports showed the locally secreted high level of IL-2 generated much less side effects than a systemically administered high dose of IL-2[31].
Double signal systems were needed in the process of inducing effective specific active antitumor T cell immune response[18]. B7-1 was an important co-stimulating molecule for tumor antigen presentation, and could provide the second signal system for T cell immune[11,32,33]. Tumors could escape from the immune surveillance of host when they were lack of it[17]. B7-1 gene-modified tumor vaccine could break the immuno-tolerance of the tumor to the host and induce effective specific antitumor immunology[32,34].
Some reports showed a single immunogene modified tumor vaccine could not induce very effective immune response because double signal systems were needed in the process[18,19] and multi-gene modified tumor vaccine could improve the immunogenicity of tumor vaccines in different ways and promote T cell immunity[18,19,35-39]. So in our experiment, we investigated the immunoprotection of single IL-2 or B7-1 gene or both IL-2 and B7-1 genes immunized mice HCC cell line Hepal-6 tumor vaccines.
In our experiment, the results showed Hepal-6 liver cancer cell line expressed a low level of B7-1 molecule and no IL-2 was secreted, and the tumorigenicity was 100%. The vaccine prepared from wild Hepal-6 cells showed very weak protection against wild tumor cell attacking. The tumor vaccine modified with IL-2 or B7-1 gene induced a stronger immunoprotective effect than the wild vaccines, and could significantly improve the survival rate and median survival time. More excitingly, the immunoprotection induced by IL-2 and B7-1 genes co-transfected tumor cell vaccine was even better than that of either of the genes. These two genes improved the immunogenicity of the liver cancer cell vaccine synergically and induced the mice to generate stronger anti-tumor immunoreactions. The immunological analysis of spleno-lymphocytes from the mice showed that IL-2 and B7-1 genes co-transfected liver cancer cell vaccine induced lymphocytes to express a significantly higher CD25 positive level and a lower CD4/CD8 ratio. This result suggested the T cells were activated. The cytotoxic analysis showed after immunization with this vaccine, specific CTLs were induced in the mice.
The results in this mouse liver cancer model demonstrated that after immunization with IL-2 and B7-1 genes co-transfected liver cancer cell vaccine, more effective systemic anti-tumor cell immunoreactions could be induce in mice than that with either of the gene modified vaccines, and the induced immuno-memory reaction could protect against the challenge of the wild parental Hepal-6 cell. The results suggest that the co-transfected gene tumor vaccine can prevent recurrence and metastasis of liver cancer.
We are grateful to Drs. Yan Zhao and Wei-Hua Bao for their advice and help in the study.
Edited by Zhang JZ and Wang XL
| 1. | Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29. [RCA] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 2. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 3. | Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical re-currence rates of hepatocellular carcinoma: a randomized trial. Lancet. 2000;356:802-807. [RCA] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 5. | Huang YH, Wu JC, Lui WY, Chau GY, Tsay SH, Chiang JH, King KL, Huo TI, Chang FY, Lee SD. Prospective case-controlled trial of adjuvant chemotherapy after resection of hepatocellular carcinoma. World J Surg. 2000;24:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 669] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Natsume A, Mizuno M, Ryuke Y, Yoshida J. Antitumor effect and cellular immunity activation by murine interferon-beta gene transfer against intracerebral glioma in mouse. Gene Ther. 1999;6:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Parmiani G, Rodolfo M, Melani C. Immunological gene therapy with ex vivo gene-modified tumor cells: a critique and a reappraisal. Hum Gene Ther. 2000;11:1269-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Arienti F, Belli F, Napolitano F, Sulé-Suso J, Mazzocchi A, Gallino GF, Cattelan A, Santantonio C, Rivoltini L, Melani C. Vaccination of melanoma patients with interleukin 4 gene-transduced allogeneic melanoma cells. Hum Gene Ther. 1999;10:2907-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Del Vecchio M, Parmiani G. Cancer vaccination. Forum (Genova). 1999;9:239-256. [PubMed] |
| 11. | Li Z, Sui Y, Jiang Y, Lei Z, Shang J, Zheng Y. Reconstruction of SEA-B7.1 double signals on human hepatocellular carcinoma cells and analysis of its immunological effect. Biochem Biophys Res Commun. 2001;288:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yamashita YI, Shimada M, Hasegawa H, Minagawa R, Rikimaru T, Hamatsu T, Tanaka S, Shirabe K, Miyazaki JI, Sugimachi K. Electroporation-mediated interleukin-12 gene therapy for hepatocellular carcinoma in the mice model. Cancer Res. 2001;61:1005-1012. [PubMed] |
| 13. | Barajas M, Mazzolini G, Genové G, Bilbao R, Narvaiza I, Schmitz V, Sangro B, Melero I, Qian C, Prieto J. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology. 2001;33:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Shi M, Wang FS, Wu ZZ. Synergetic anticancer effect of combined quercetin and recombinant adenoviral vector expressing human wild-type p53, GM-CSF and B7-1 genes on hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9:73-78. [PubMed] |
| 15. | Dan Q, Sanchez R, Delgado C, Wepsic HT, Morgan K, Chen Y, Jeffes EW, Lowell CA, Morgan TR, Jadus MR. Non-immunogenic murine hepatocellular carcinoma Hepa1-6 cells expressing the membrane form of macrophage colony stimulating factor are rejected in vivo and lead to CD8+ T-cell immunity against the parental tumor. Mol Ther. 2001;4:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Tatsumi T, Takehara T, Kanto T, Kuzushita N, Ito A, Kasahara A, Sasaki Y, Hori M, Hayashi N. B7-1 (CD80)-gene transfer combined with interleukin-12 administration elicits protective and therapeutic immunity against mouse hepatocellular carcinoma. Hepatology. 1999;30:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellström I, Hellström KE. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 352] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Paul DB, Barth RF, Yang W, Shen GH, Kim J, Triozzi PL. B7.1 expression by the weakly immunogenic F98 rat glioma does not enhance immunogenicity. Gene Ther. 2000;7:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Buggins AG, Lea N, Gäken J, Darling D, Farzaneh F, Mufti GJ, Hirst WJ. Effect of costimulation and the microenvironment on antigen presentation by leukemic cells. Blood. 1999;94:3479-3490. [PubMed] |
| 20. | Heike Y, Takahashi M, Ohira T, Naruse I, Hama S, Ohe Y, Kasai T, Fukumoto H, Olsen KJ, Podack EE. Genetic immunotherapy by intrapleural, intraperitoneal and subcutaneous injection of IL-2 gene-modified Lewis lung carcinoma cells. Int J Cancer. 1997;73:844-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Atkins MB. Interleukin-2: clinical applications. Semin Oncol. 2002;29:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Herberman RB. Cancer immunotherapy with natural killer cells. Semin Oncol. 2002;29:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Guarini A, Riera L, Cignetti A, Montacchini L, Massaia M, Foa R. Transfer of the interleukin-2 gene into human cancer cells induces specific antitumor recognition and restores the expression of CD3/T-cell receptor associated signal transduction molecules. Blood. 1997;89:212-218. [PubMed] |
| 24. | Kim JH, Gong SJ, Yoo NC, Lee H, Shin DH, Uhm HD, Jeong SJ, Cho JY, Rha SY, Kim YS. Effects of interleukin-2 transduction on the human hepatoma cell lines using retroviral vector. Oncol Rep. 1999;6:49-54. [PubMed] |
| 25. | Vanhaesebroeck B, Mareel M, Van Roy F, Grooten J, Fiers W. Expression of the tumor necrosis factor gene in tumor cells correlates with reduced tumorigenicity and reduced invasiveness in vivo. Cancer Res. 1991;51:2229-2238. [PubMed] |
| 26. | Tatsumi T, Takehara T, Katayama K, Mochizuki K, Yamamoto M, Kanto T, Sasaki Y, Kasahara A, Hayashi N. Expression of costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on human hepatocellular carcinoma. Hepatology. 1997;25:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Qian SB, Chen SS. Transduction of human hepatocellular carcinoma cells with human alpha-interferon gene via retroviral vector. World J Gastroenterol. 1998;4:210-213. [PubMed] |
| 28. | Hájková R, Indrová M, Jandlová T, Bubeník J, Reinis M. Interleukin 2 gene therapy of surgical minimal residual tumour disease: characterization of cytolytic effector cells from tumour progressors and regressors. Folia Biol (Praha). 1999;45:227-231. [PubMed] |
| 29. | Mizuno H, Yanoma S, Nishimura G, Hattori S, Ito T, Okudera K, Tsukuda M. Therapeutic efficiency of IL-2 gene transduced tumor vaccine for head and neck carcinoma. Cancer Lett. 2000;152:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Palmer K, Moore J, Everard M, Harris JD, Rodgers S, Rees RC, Murray AK, Mascari R, Kirkwood J, Riches PG. Gene therapy with autologous, interleukin 2-secreting tumor cells in patients with malignant melanoma. Hum Gene Ther. 1999;10:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Tagawa M. Cytokine therapy for cancer. Curr Pharm Des. 2000;6:681-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Takahashi T, Hirano N, Takahashi T, Chiba S, Yazaki Y, Hirai H. Immunogene therapy against mouse leukemia using B7 molecules. Cancer Gene Ther. 2000;7:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Yang G, Mizuno MT, Hellström KE, Chen L. B7-negative versus B7-positive P815 tumor: differential requirements for priming of an antitumor immune response in lymph nodes. J Immunol. 1997;158:851-858. [PubMed] |
| 34. | Antonia SJ, Extermann M, Flavell RA. Immunologic nonresponsiveness to tumors. Crit Rev Oncog. 1998;9:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Barnard AL, Farzaneh F, Gäken J, Darling D. Local versus systemic interleukin-2: tumor formation by wild-type and B7-1-positive murine melanoma cells. Cancer Gene Ther. 2000;7:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Fujiwara H, Yamauchi N, Sato Y, Sasaki K, Takahashi M, Okamoto T, Sato T, Iyama S, Koshita Y, Hirayama M. Synergistic suppressive effect of double transfection of tumor necrosis factor-alpha and interleukin 12 genes on tumorigenicity of Meth-A cells. Jpn J Cancer Res. 2000;91:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Hurwitz AA, Townsend SE, Yu TF, Wallin JA, Allison JP. Enhancement of the anti-tumor immune response using a combination of interferon-gamma and B7 expression in an experimental mammary carcinoma. Int J Cancer. 1998;77:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Mazzocchi A, Melani C, Rivoltini L, Castelli C, Del Vecchio M, Lombardo C, Colombo MP, Parmiani G. Simultaneous transduc-tion of B7-1 and IL-2 genes into human melanoma cells to be used as vaccine: enhancement of stimulatory activity for autolo-gous and allogeneic lymphocytes. Cancer Immunol Immunother. 2001;50:199-211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |