INTRODUCTION
Gastric cancer is common in China[1-14]. For decades, a goal of cancer researchers is to be able to immunize patients with their own tumor tissues or tumor associated antigens after surgical operation to stimulate their immune response. The benefits of this aim include host immune surveillance to eliminate the metastatic cells and prevent its relapse. Many tumor associated antigens defined by monoclonal antibodies have been successfully applied clinically in detecting different tumors[15,16]. However, only a few of the genes for these markers have been cloned[17,18], primarily because the epitopes usually are not protein or not linear in amino acid sequence.
MAb PD4 has been derived from mouse immunized with human gastric cell line MGC803 and could specially react with some tumor cells[19]. Our previous studies showed that MAb PD4 could induce apoptosis of MGC803 cells[20], inhibit both the growth of ras transformed cell line Rat3-3 and the tumorigenesis in nude mice[21], suggesting that the antigen recognized by MAb PD4 could be associated with cancerous development. Obviously the critical step in investigating the molecular mechanisms involved in the antigen is to isolate its cDNA.
As it was shown previously that PD4 worked well in Western blot in which the recognized protein was around 40 kilo-Dalton in molecular weight[21]. First, we screened the cDNA library constructed with MGC803 cells by MAb PD4 as probes. Unfortunately, the positive clones identified with MAb PD4 were reacted with unrelated antibodies. We tried with other different tumor expression cDNA libraries and also did not get any specific clones. Then, immunoprecipitation was performed and the target molecule was identified to be a membrane protein of M. hyorhinis by N-termial amino acid residues sequencing. The membrane protein was intensively verified with Western blot by eliminating M. hyorhinis from MGC803 cells and by infecting M. hyorhinis-free Hela cells. Therefore, the actions involved in MAb PD4 were possibly mediated by p37 protein or M. hyorhinis.
p37 is a membrane protein of M. hyorhinis located on the outside of the cell membrane. It contains 1209 nucleotides and encodes 403 amino acid residues[22]. An analysis of the protein sequence has revealed that p37 has a 41% similarity to a periplasmic binding-protein-dependent transport system found in Gram-negative bacteria[22]. Thus, p37 is thought to be part of a high affinity transport system from M. hyorhinis. Also, there are lines of evidence indicating the linkage between p37 or M. hyorhinis and cancer[23-25]. For example, antibodies against p37 could inhibit the invasive potential of FS9 cells and cause malignant cells to revert to a more normal behavior.
In this study, we identified the antigen recognized by MAb PD4, which was previously considered as an antibody against cancer. The full gene encoding the antigen was cloned and expressed successfully in Escherichia coli (E. coli) after site-directed mutation of the seven codes tryptophan TGA into universal codes tryptophan TGG and demonstrated that p37 could bind directly to tumor cell AGS. Considering the association between p37 and tumor development and invasion, this work provides a basis for further investigation of the pathogenic role of p37 involved in M. hyorhinis infection.
MATERIALS AND METHODS
Cell culture and regents
Human gastric cancer cell lines MGC803 and AGS, human ovarian cancer cell line HeLa, expression plasmid pGEX-4T-1, E. coli BL21(DE3) and MAb PD4 were all kept in our laboratory. Site-directed mutation kit was purchased from Promega Corp. Anti-glutathione-S-transferase (GST) mouse antibody, goat anti-mouse antibody conjugated with tetramethyl rhodaine isothiacy-anate (TRITC), 3,3’-diaminobenzidine (DAB), isopropylthiogalactoside (IPTG) were from Sigma. All primers for PCR and site-directed mutation were synthesized by Sangon Corp. (Shanghai, China). Various restriction endonucleases were products of New England BioLabs (NEB). RPMI1640 and F12K medium were from GIBCO BRL. RNA extraction kit was from Invitrogen Corp.
cDNA library construction, screening and clone identification
mRNA purification and poly(A)+ mRNA enrichment were processed with a messenger RNA isolation kit (Strategene Corp.) from 5 × 107 MGC803 cells. A cDNA library was prepared and packaged with ZAP Express cDNA Gigapack III gold cloning kit (Strategene Corp.) according to the manufacture’s instruction. The library quality was determined with its diversity and the average size of the inserted cDNA fragments. The non-amplified library was plated and transferred to nitrocellulose filters. Initial screening was performed with 1 μg·mL-1 MAb PD4 (diluted in phosphate-buffered saline containing 1% bovine serum albumin). The filters were then washed in phosphate-buffered saline (PBS) and bound MAb PD4 was detected by alkaline phosphatase coupled to sheep anti-mouse antibodies followed by the mixture solution of nitro blue tetrazolium and bromochloroindolylphosphate. The positive bacteriophages were subcloned and the positive individual clones were checked with unrelated antibodies for its specificity.
Preparation of MGC803 cell membrane proteins
2 × 108 MGC803 cells were frozen and thawed repeatedly for 4-5 times in phosphate-buffered saline containing 1 mmol·L-1 PMSF, then centrifuged at 4000 × g for 30 min at 4 °C. The supernatant was collected, centrifuged at 100000 × g for 1 h at 4 °C. The precipitated membrane debris was suspended with 1-2 mL lysis buffer (20 mM Tris-HCl pH7.5, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 5% sodium deoxycholate, 1 mM PMSF, 2 μg·mL-1 aprotinin), shaken for 30 min at 4 °C, then centrifuged at 100000 × g for 1 h at 4 °C. The supernatant was analyzed by Western blot with MAb PD4.
Purification and identification of the antigen protein
The lysates from MGC803 cells were immunoprecipitated for overnight at 4 °C by MAb PD4 coupled with protein A-sepharose 4B beads (Sigma). The normal mouse IgG was used as control. The immunoprecipitated beads were washed three times with lysis buffer and subjected to 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The gel-separated proteins were transferred onto PVDF membranes. A piece cut off from the membrane was used for Western blot with MAb PD4 and the left transferred membrane was stained with ponceau S. After the identification, the corresponding band with the Western blot was cut off and the sequence of N-terminal amino acid residues was performed in Life Science Center of Peking University.
Further identification of the antigen from mycoplasma infected cells
When M. hyorhinis-free HeLa cells grew to appropriate confluence, the conditional medium from cultured MGC803 cells, which reacted with MAb PD4, was added into the cultured HeLa cells system. After 72 h incubation, total proteins were extracted from HeLa cells with lysis buffer (1% Triton X-100/PBS, 1 mmol·L-1 PMSF) and Western blot was performed with MAb PD4 as first antibody. Moreover, cultured MGC803 cells were treated with BM cyclin antibiotics for 3 weeks (Roche, Mannheim, Germany) which were recognized as the most effective against mycoplasma infection[26]. Then the total proteins of MGC803 cells were extracted, followed by Western blot as above.
Cloning, site-directed mutations and sequencing of p37 gene
3 mL supernatant from cultured MGC803 cells infected with M. hyorhinis was collected, centrifuged at 12000 × g for 5 min, rinsed once with PBS, dissolved in 30 μL ddH2O, then boiled for 5 min, centrifuged for 5 min at 10000 × g. 2 μL supernatant was used as the template for PCR to amplify p37 gene. Upstream primer 5’-aatcggatcc gaggtagcttttatgctc-3’ (including BamH I site) and downstream primer 5’-aaagaattctcatttaatggcttttt c-3’ (including EcoR I site) were synthesized by Sangon Co. (Shanghai. China). The PCR program was consisted of 30 cycles at 94 °C for 1 min, at 48 °C for 1 min, at 72 °C for 90 s, and at 72 °C for 10 min for the final extension. The PCR product was inserted into a pBluescript vector after digestion with endonucleases BamH I and EcoR I. The seven tryptophan TGA codes were mutated into universal codes tryptophan TGG using mutation vector pALTER-1 according to the instructions of the site-directed mutation kit (Promega) and verified by DNA sequencing. The 5’phosphated mutated primers were as follows: (1) 5’-p(AGGTCAATGGGATAAAAGTA)-3’, (2) 5’-p(GCAAGTTGGACTGATGAAAATCATAAGTGGAATGGTAATG)-3’, (3) 5’-p(GGAATGATTTGGATAAAAGGTAATG)-3’, (4) 5’-p (AAAAAGCTTGGAATGATAAAGATTGGAATACATTTAGAAATTTT)-3’, (5) 5’-p(GGTTCTTTTGCTTGGACACATAACA)-3’. The bold letters were changed from A.
Expression, purification and identification of GST-P37 fusion protein
The recombinant vector pGEX-4T-p37 was constructed through inserting the mutated p37 into plasmid pGEX-4T-1, then it was introduced into E. coli BL21(DE3). The transformed bacteria were induced with 0.1 mM IPTG to express the fusion protein at 30 °C overnight. Then the induced bacteria were collected and lysed by ultrasonocation. After centrifugation, the supernatant was incubated with glutathione-sepharose-4B and binding protein was eluted with elution buffer (50 mM Tris-HCl, pH8.0, 15 mM reduced glutathione). The GST-p37 protein in elution buffer was tested by SDS-PAGE and identified by Western blot after concentrated with sucrose and dialyzed with PBS.
Binding assay with immunofluorescence microscopy
AGS cells were seeded into 6-well culture plates in F-12K medium and crept on cover slides for 24 h. Then the cells were washed twice by PBS. The GST-p37 and GST proteins (10 µg·mL-1) dissolved in serum-free F-12K medium were added respectively. After incubation for 1 h, cells were gently washed 3 times by PBS and fixed for 15 min with freshly prepared 4% paraformaldehyde. Then the cells were washed twice by PBT buffer (PBS with 0.1% bovine serum albumin and 0.01% Tween 20) and blocked with 10% normal goat serum for 1 h at 37 °C. Anti-GST antibodies were added as the first antibody and incubated for 1 h at 37 °C. After the cells were washed twice for 5 min each by PBT buffer, TRITC-conjugated goat anti-mouse IgG antibodies were used as secondary antibody to detect bound GST-p37 and GST proteins. The cells were washed twice with PBT buffer and briefly rinsed with water before mounted onto slides for observation under fluorescence microscopy.
RESULTS
cDNA library construction, screening and identification
We obtained 2 μg poly (A)+ enriched mRNA for synthesizing the cDNA library. Double-stranded cDNA was generated by means of “nick-translation”. EcoR I adapters were ligated to the double-stranded cDNA, which was then digested with XhoI and size selected. Only the cDNA molecules larger than 500 bp were collected and ligated into ZAP expression vectors. The diversity of the primary library was 1.2 × 106 pfu. The recombinant rate was 9/9, and the average size of cDNA inserts was about 1.5 kb. A total of 106 non-amplified library clones were screened with MAb PD4 and the positive clones were confirmed with unrelated antibodies as controls. Anyhow no specific positive clone reacted with MAb PD4 was obtained.
Purification of the antigen recognized by MAb PD4
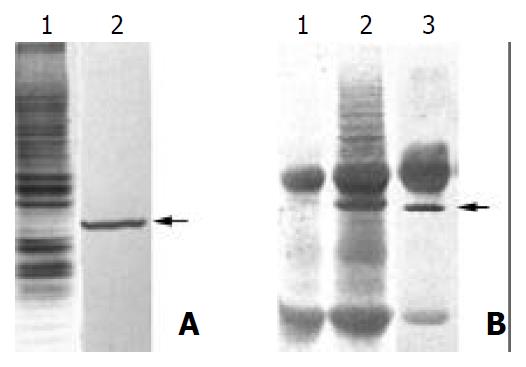
First, the total membrane protein from MGC803 was analyzed by Western blot with MAb PD4, and a specific 40 kilo-Dalton band could be found (Figure 1A). Then immunoprecipitation was performed and the protein complex was subjected to 12% SDS-PAGE. The gel-separated proteins were analyzed with Western blot, and the target band was cut off from the membrane (Figure 1B). The sequence of N-terminal amino acid residues revealed that 16 amino acid residues (CSNTGVVKQEDVSVSQ) were completely identical with protein p37 from M. hyorhinis, which suggested the antigen recognized by MAb PD4 was from mycoplasma, not from tumor cells, and the PD4 was a MAb against M. hyorhinis.
Figure 1 Purification of the antigen by MAb PD4.
(A) 1. SDS-PAGE and Coomassie blue staining of the membrane proteins extracted from MGC803 cells; 2. Western blot analysis of the membrane proteins extracted from MGC803 with MAb PD4, the arrow indicating the target protein of MAb PD4. (B) 1. SDS-PAGE analysis of immunoprecipited protein complex bound with normal mouse IgG. (B) 2. SDS-PAGE analysis of immunoprecipited protein complex bound with MAb PD4. (B) 3. Western blot analysis of immunoprecipited protein complex bound with MAb PD4, arrow indicating the target protein of MAb PD4.
Further identification of the antigen with mycoplasma infected cells
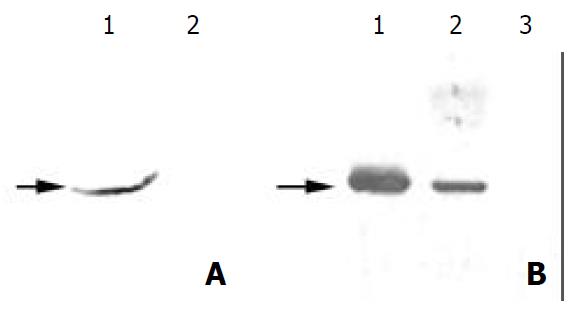
If the antigen was really derived from M. hyorhinis, it should be transferred along with M. hyorhinis infection and also would disappear when the infected mycoplasma was cleared away from host cells. HeLa cells, which were mycoplasma-free and not reactive with PD4, were treated with conditional medium from M. hyorhinis infected cell MGC803. After 72 h treatment, the totally extracted cell protein could react with PD4 (Figure 2A). Meanwhile, the reacting band with PD4 disappeared after MGC803 cells were treated by BM cyclin antibiotics. This result indicated that the antigen of MAb PD4 was a protein from M. hyorhinis.
Figure 2 Further identification of the antigen from mycoplasma infection.
(A) 1. Western blot analysis of the total protein from Hela cells with MAb PD4, which was treated with cultured MGC803 medium. (A) 2. Western blot analysis of the total pro-tein from untreated HeLa cells with MAb PD4. (B) Western blot analysis of the total proteins from MGC803 cells treated with BM cyclin antibiotics differently. Lane 1: untreated, lane 2: treated for two weeks, and lane 3: treated for three weeks. As indicated by the arrow, the band reacted with MAb PD4 disappeared gradually following the treatment.
Cloning, expression and purification of GST-P37 protein
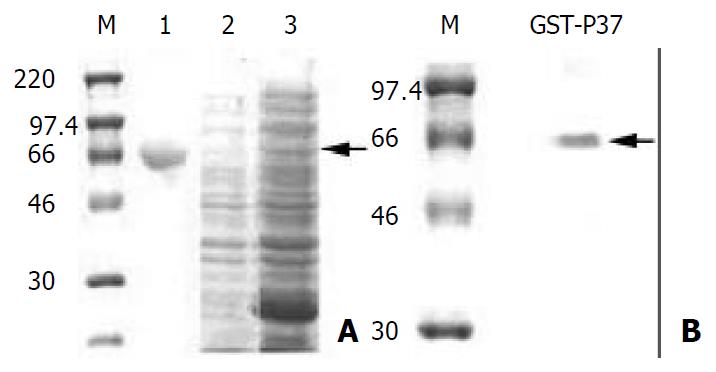
The results above suggested that the effect of MAb PD4 on tumor cells was mediated by p37 or M. hyorhinis. To investigate the molecular mechanisms of the effect, the gene p37 was cloned and mutated by site-directed mutagenesis successfully. After checked by DNA sequencing, the full length p37 was expressed as fusion protein GST-p37 and it could react with PD4 specifically by Western blot (Figure 3).
Figure 3 Analysis of GST-p37 with SDS-PAGE and Western blot.
(A) Coomassie blue staining of the SDS-PAGE gel. M pro-tein standards. lane 1: protein of BSA, lane 2: total proteins from un-induced bacteria, lane 3: total proteins from IPTG in-duced bacteria, the arrow indicating GST-p37 band. (B) West-ern blot analysis of purified protein with MAb PD4, the arrow indicating GST-p37 band.
Binding assay
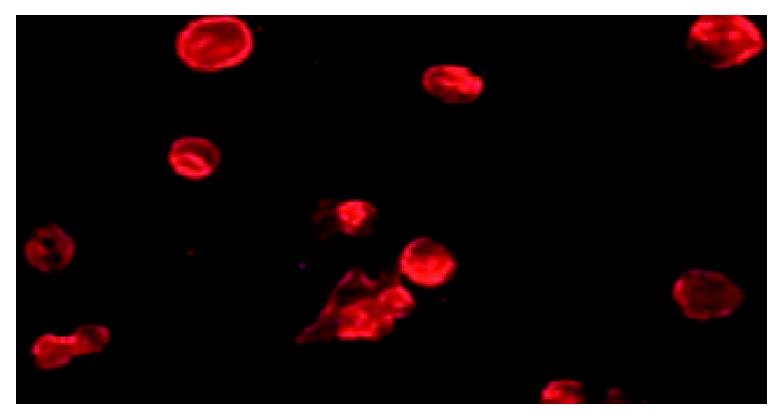
Because p37 is a dominant protein component of M. hyorhinis located on the outside of the cell membrane, we hypothesized that p37 was capable of mediating M. hyorhinis binding to host cells. In current study, we first demonstrated that p37 could directly bind to gastric cell line AGS (Figure 4), which provided the basis for further related investigation of p37.
Figure 4 p37 binding AGS cells assay.
Observation of the immunofluorecence under microscopy, which showed GST-p37 protein binding to AGS cells, whereas GST protein has no such binding ability (data not shown).
DISCUSSION
Our study indicated that the antigen recognized by MAb PD4, which was derived from mice immunized with gastric tumor cell line MGC803, was a protein from M. hyorhinis. This result suggests that the actions of MAb PD4, which we showed before, are mediated possibly by p37 protein or M. hyorhinis. Mycoplasmas are a heterogeneous group of the smallest organisms capable of self-replication that can cause a wide variety of diseases in animals. Some mycoplasmas cause respiratory or urogenital diseases in humans[27,28], but others chronically colonize on respiratory and urogenital tracts without apparent clinical significance. There have been some discrete reports about the correlation between mycoplasma infection and cancer since last century. In 1960s, two studies reported that infection of mycoplasma (M. orale and one unspeciated) caused chromosomal changes[29]. Other researches in the 1980s reported that an arthropod spiroplasma could rapidly transform mouse and monkey cells[30]. However, investigating the possible association between mycoplasma infection and carcinogenesis did not become more active until Tsai et al[30,31] reported that continuous infection of M. penetrans or M. fermentans could lead to multiple stage of malignant transformation of murine embryonic C3H cells, accompanied by abnormal karyotypes and some oncogene upregulation.
Also, there are documents indicating the linkage between p37 protein or M. hyorhinis and cancer. One is that elevated tumor invasion, as suggested by a leukocyte adherence inhibition (LAI) response, correlates with the presence of M. hyorhinis in patients with lung cancer, colon cancer, and breast cancer[32]. Furthermore, two independent research groups showed that p37 on the surface of FS9 mouse fibrosarcoma cells correlated with a highly invasive phenotype, as measured in an in vitro cellular invasion assay[22,23]. Antibodies against p37 inhibited the invasive potential of FS9 cells in the assay in vitro[23]. Using Abercrombie’s confronted explant assay, it was shown that antibodies against p37 caused malignant cells to revert to a more normal behavior[24]. More recently, p37 antibodies were reported to reduce the lung metastasis of colon cancer in nude mouse models[25]. These results, taken together, suggest that M. hyorhinis infection causes enhanced tumor invasion and correlates with tumorigenesis, and that p37 is an essential component of this phenomenon.
In order to further investigate the pathogenic role of p37 in M. hyorhinis infection, we cloned the full p37 gene and expressed it successfully in E. coli. Eighty percent of the expressed GST-p37 fusion protein was soluble at the inducing condition (IPTG 0.1 mM, 30 °C overnight). Western blot analysis indicated the expressed p37 protein was intact. As p37 is a dominant protein component of M. hyorhinis located on the outside of the cell membrane, we hypothesize that p37 may be capable of mediating M. hyorhinis binding to host cells. In the current study, we first demonstrated that p37 could directly bind to gastric cell line AGS, which provided the basis for further related investigation of p37.
M. hyorhinis contamination is very common in routine cell culture and mycoplasma may be more prone to stimulate immune response when mice are immunized with tumor cells infected with mycoplasma. Thus, the obtained monoclonal antibodies are possibly raised to mycoplasma, especially when the infected tumor cells are used as both immunogen and target cells for screening positive clones. This should draw more attention during preparing monoclonal antibodies. Interestingly, when we recently performed immunohistochemistry with PD4 to detect M. hyorhinis infection in paraffin embedded carcinoma tissues, the results indicated that positive rates in gastric carcinoma, esophageal cancer, colon carcinoma and lung cancer were around 50%, but they were less than 25% in other gastric diseases, such as chronic superficial gastritis, gastric ulcer and intestinal metaplasia[33]. Meanwhile, we have also isolated M. hyorhinis successfully from human gastric cancer tissues by direct culture (data not published). These results strongly suggest an association between mycoplasma infection and tumorigenesis. Undoubtedly, the molecular mechanism of p37 action on tumor cells needs to be intensively investigated.