Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.79
Revised: September 11, 2002
Accepted: October 12, 2002
Published online: January 15, 2003
AIM: To construct interleukin-2 gene-modified human hepatocyte line (L-02/IL-2) and investigate the changes of the function of liver cells and IL-2 secretion in culture with microcarrier, laying the foundation for further experimentation on hepatocyte transplantation.
METHODS: hIL-2 gene was transduced into L-02 hepatocytes by recombinant retroviral vector pLNCIL-2, and the changes of morphology and clonogeneicity rate of the transduced cells were observed, the secretion levels of hIL-2 in cultural supernatant were detected by ELISA and NeoR gene was amplified by PCR. The growth of L-02/IL-2, the special biochemistry items and the levels of IL-2 were detected after cultivation with microcarrier.
RESULTS: The clonogeneicity rate of the L-02/IL-2 cells was lower than that of L-02/Neo cells and L-02 cells. The levels of hIL-2 could reach 32000 pg/106 cells per day and kept secreting for more than ten weeks. NeoR gene segment was respectively obtained by PCR from both L-02/IL-2 and L-02/Neo cell’s genomic DNA. At the 6th day in culture with microcarrier, the matrix-induced liver cell aggregates were formed, the number of alive L-02/IL-2 cell were 16.8 ± 0.53 × 106/flask and the levels of ALB and UREA were 52.54 ± 1.28 mg/L and 5.29 ± 0.17 mmol/L, respectively. These data had not significantly changed as compared with those of L-02 cells (P > 0.05); However, the levels of IL-2 in IL-2/L-02 cells remarkably exceeded that in L-02 cells in the whole culture process (P < 0.001).
CONCLUSION: The IL-2 gene-modified hepatocyte line has been successfully constructed. The L-02/IL-2 cellular aggregates cultured with microcarrier have a high capacity of IL-2 production as well as protein synthesis and amino acid metabolism.
- Citation: Tang NH, Chen YL, Wang XQ, Li XJ, Yin FZ, Wang XZ. Construction of IL-2 gene-modified human hepatocyte and its cultivation with microcarrier. World J Gastroenterol 2003; 9(1): 79-83
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/79.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.79
Gene therapy has become an important therapeutic alternative in recent years, thanks to the growing improvement of gene-transduction techniques in eukaryotic cells. There are a large number of proteins expression in hepatocyte with high levels, and many genes that are involved in metabolism also express in hepatocytes, so hepatocyte is one of the crucial tools for gene therapy[1], for example, the therapeutic implement for patient with hyperbilirubinemia by hepatocyte transplantation after gene modification in vitro, which was approved by FDA[2].
As an important approach for inhibiting the growth of tumor, immune therapy of cytokines further broadens the prospect of clinical application of hepatocytes and gene therapy of cytokines. Recent studies have demonstrated the feasibility of cytokine gene transferrence to enhance the antitumor activities of host immune cell[3]. With regards to therapy for hepatocarcinoma, there are many good results obtained from the study of hepatocarcinoma cells modified by cytokine genes[4-11], especially using IL-2 gene modified hepatocarcinoma cells that conquered the substantial toxicity from administration of high doses of IL-2[9,12]. However, few studies focus on the antitumor immune function of hepatocyte transduced with cytokine gene in hepatocyte transplantation. In this study, IL-2 gene was transduced into human hepatocyte line L-02 by recombinant retroviral vector. The experiments of its biologic activities and cultivation with microcarrers were performed, laying the foundation for further experimentation on hepatocyte transplantation.
Human hepatocyte line L-02, amphotropic packaging cell PA317 and mouse fibroblast cell line NIH3T3 were purchased from Shanghai Cell Biology Institute, Chinese Academy of Science and grown in DF medium (DMEM: Ham’s F12 = 3:1) containing 100 mL·L-1 fetal calf serum, penicillin 1 × 105 U·L-1 and streptomycin 100 mg·L-1. The cells were kept at 37 °C in a 50 mL/LCO2 humidified atmosphere and subcultered from one to three when the cells proliferated into a full monolayer. Recombitant retroviral vector, LNCX and LNCIL-2, were kindly provided by Prof. Joo Hang Kim from Yonsei University in Korea. Plasmid extraction and purification kit, Wizard® Plus SV Minipreps DNA, and transfection kit TransFastTM were from Promega. hIL-2 ELISA kit was purchased from Jingmei Biological Engineering, Shenzhen. Microcarrier Cytodex3 was from Pharmasia. Poly-HEMA, DMEM and Ham’s F12 medium were the products of Sigma. Calf serum were purchased from Four Season Green Biological Co., Hangzhou, China.
Construction of recombitant retrovirus producer cell line Transformation of recombitant retroviral vector was performed as previously described[13]. The extraction and purification of the product was operated according to the manufacturer’s protocol. The purified products were quantitated by spectrophotometer (DU 640), digested with Hind III at 37 °C for 2 h and identified by eletrophoresis through a 10 g/L formaldehyde agarose gel. The amphotropic packaging cells PA317 were plated into 12-well plates and cultured till nearly 60%-70% confluence. Then LNCX or LNCIL-2 transduction to PA317 cell line was made with lipo-transfection technique. The PA317/Neo and PA317/IL-2 cell clones which produced pLNCX and pLNCIL-2 were respectively selected by G418 and subcultured for amplification in G418-free DF media for 24 h. Furthermore, the supernatant of above cells containing recombitant retrovirus were collected, and the retrovirus titer was detected by NIH3T3 cells according to that described[14], quantitated highest titer was kept at -70 °C.
Transduction of IL-2 gene into the hepatocyte cell line The L-02 hepatocyte line were grown to a confluence of 60%-70% in 24-well plate. The supernatant were discarded and replaced with 1 mL recombitant retroviral supernatant supplemented with 8 µg of Polybrene. Two hours later, 2 mL fresh DF media containing 800 mg/L were added and cultured at 37 °C for 24 h. The whole process of the cell clone against G418 selection lasted 15 days followed by amplified cultivation.
Growth of transfected cells The growth of transfected cells were observed and photographed, total of 3 × 103 L-02, L-02/Neo and L-02/IL-2 cells were put into the media respectively with a final volume of 200 µL, and then plated on a 96-well plate and incubated at 37 °C in 5 mL/LCO2 humidified atmosphere for 7 days. Each group had six wells. The assay of cell proliferation was performed by MTT every 24 h. Briefly, 20 µL of 5 g/L MTT were added into each well and cultured for another 4 h. The supernatant was discarded and replaced with 200 µL of dimethyl sulfoxide (DMSO). When the crystals were dissolved, the absorbance (A) value of the slides was read at 490 nm. In addition, 1 × 103 cells per well of the three kinds of cell were plated into 24-well plate in 1 mL media and cultured for 7 days, respectively. The colonogeneicity rate (CR) of transferred cells were calculated by using the following equation: CR = (average clones per well/1000) × 100%.
NeoR gene analysis of the transfected cells by PCR The total genomic DNA were extracted from 1.0 × 106 of L-02, L-02/Neo and L-02/IL-2 cells in 200 mL TE buffer respectively, then quantitated by spectrophotometer and digested with BamHI. The NeoR gene was amplified under following conditions: denaturation at 94 °C for 5 min followed by 94 °C 1 min, 62 °C 1 min and 72 °C 1 min 15 s for 30 cycles. The sequence of NeoR gene primers and length of PCR products were as follows: forward-5’-CAAGATGGATTGCACGCAGG-3’ and reverse-5’-CCCGCTCAGAAGAACTCGTC-3’, size, 790 bp. For analysis, 10 µL of reaction product were checked in 10 g/L agarose gel with ethidium bromide staining and followed by camera photographing.
Detection of the levels of hIL-2 secreted by the transduced cells The supernatant of 1.0 × 106 of L-02, L-02/Neo and L-02/IL-2 cells that cultured in flask for 24 h were obtained and stored at -70 °C after centrifugation. L-02/IL-2 cells were especially cultured for 10 wk and the supernatants were collected every week. The levels of hIL-2 were measured by ELISA according to the manufacturer’s protocol.
Cultivation with microcarriers All glassware with which Cytodex3 came into contact, should be siliconized before use. The hydration of Cytodex3 was carried out as the protocol. Briefly, 200 mg Cytodex3 were dipped in Ca2+ and Mg2+-free PBS in a siliconized container overnight and sterilized by autoclaving, then replaced with fresh DF media; The flasks were covered with 0.1 mL/cm2 120 g/L Poly-HEMA dissolved in ethanol and then air-dried sterilizedly. The hydrated Cytodex3, 20 mg a flask, along with L-02 and L-02/IL-2 cells, 3.0 × 106 a flask in 2 mL media, were seeded into Poly-HEMA covered flasks, respectively. Each group had five bottles. They were all cultured in incubator at 37 °C for 4 h following shakes twice for 1 min every 4 h, the media were replaced every 24 h, and the supernatants were kept at -70 °C after centrifugation at 1000 r/min.
Morphologic observation and cell proliferation The culture process was observed and photographed. The cell samples at 6th day were fixed by 25 g/L glutaraldehyde and observed by HU-12A electronmicroscope. The proliferation was performed as follows: (1) 100 µL cell suspension were taken out from every flask at day 2, 4, 6, 8 and 10 in culture and added into a new flask without Poly-HEMA; (2) When all microcarriers went down, the supernatants were discarded, 100 µL 2.5 g/L trypsinase were added and cultured at 37 °C for 5 min; (3)After 100 µL DF media were added for termination of trypinization, the flasks were put up slowly with all microcarriers anchored to the bottom of the flask, and the supernatant were rapidly dipped out for cell calculation with trypan blue dye exclusion method.
Measurement of biochemical items and IL-2 in the supernatant With γ calculator (SN-682), the concentration of human ALB was detected by RIA kit from North Biotechnique Institute, Beijing, others as UREA (BUN × 2.14), AST and LDH were measured by biochemical autoanalyzer (CX △ 7, Beckman).
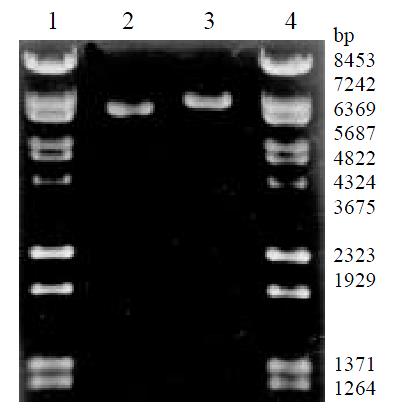
The amplified products of LNCX and LNCIL-2 were digested with Hind III, and then checked with 20 g/L agarose gel electrophoresis (Figure 1). The length of LNCX (6620 bp) and LNCIL-2 (7293 bp) were identical with that predicted, showing the success of amplification, extraction and purification.
21 and 25 anti-G418 clones appeared after PA317 cells were transduced by LNCX and LNCIL-2 and cultured in media containing 800 mg/L of G418 for 2 weeks. 10 clones selected were performed amplified culture, respectively. The titer of retrovirus of all collected supernatants was between 5.4 × 107 cfu/Land 1.4 × 109 cfu/L.
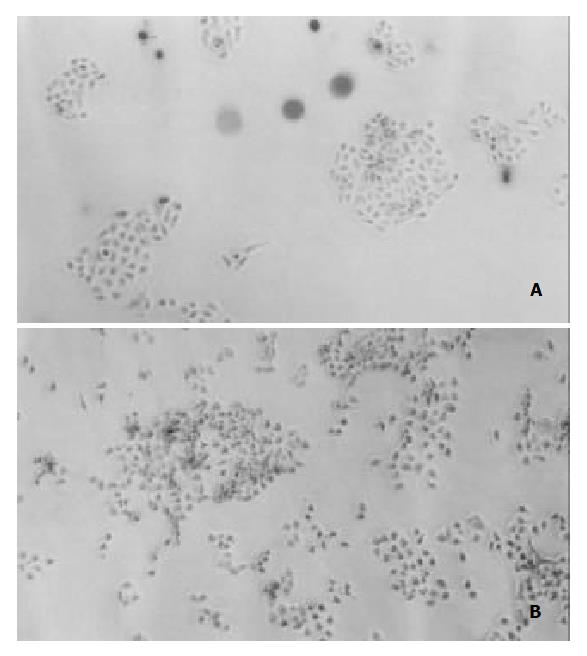
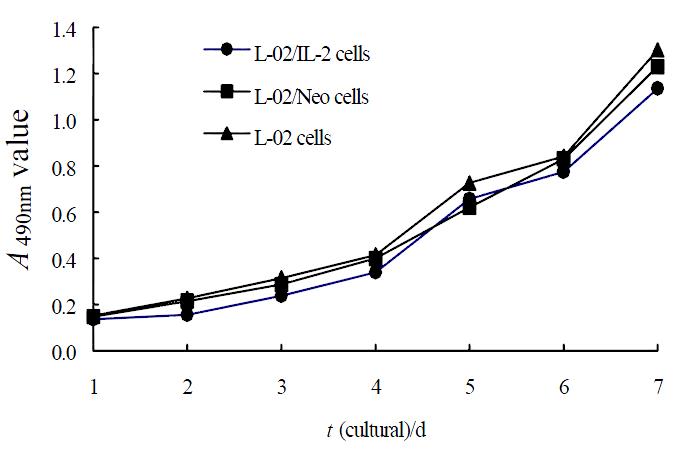
Under the light microscope, L-02 cells were seen flake-like growth (Figure 2A), L-02/Neo and L-02/IL-2 cells represented the trend of island-like growth with clear margin (Figure 2B). The growing speed of L-02/IL-2 cells was slightly slower than that of L-02 and L-02/Neo cells (Figure 3). Clonogeneicity rate of the L-02/IL-2 cells were significantly lower than those of L-02/Neo and L-02 cells (P < 0.01) (Table 1).
| No. of repeated wells | L-02/IL-2 | L-02/Neo | L-02 |
| 1 | 124 | 148 | 150 |
| 2 | 135 | 144 | 157 |
| 3 | 133 | 154 | 144 |
| 4 | 144 | 141 | 153 |
| 5 | 128 | 166 | 162 |
| 6 | 123 | 152 | 156 |
| Mean value | 131.2 (13.1%)b | 150.8 (15.1%) | 153.7 (15.4%) |
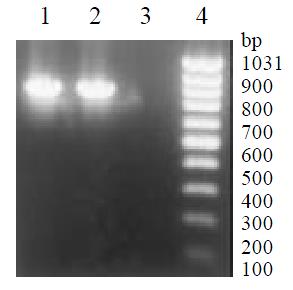
NeoR gene segment (790 bp) was amplified by PCR from genomic DNA of L-02/Neo and L-02/IL-2 cells and tested with 20 g/L agarose gel electrophoresis, but none from L-02 cells (Figure 4). These suggested that LNCX and LNCIL-2 were successfully integrated into the genome of L-02 cells.
After IL-2 transduction and G418 selection using LNCX and LNCIL-2 retroviral vector, maximal amount of IL-2 production in L-02/IL-2 cells was 32000 pg/106 cells·24 h, remarkably exceeding 56 pg/106 cells·24 h in L-02 cells and 48 pg/106 cells·24 h in L-02/Neo cells. Moreover, more than ten weeks later the levels of hIL-2 could rise to 27500 pg/106 cells·24 h.
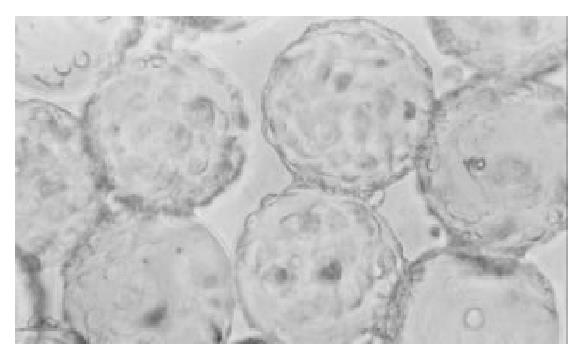
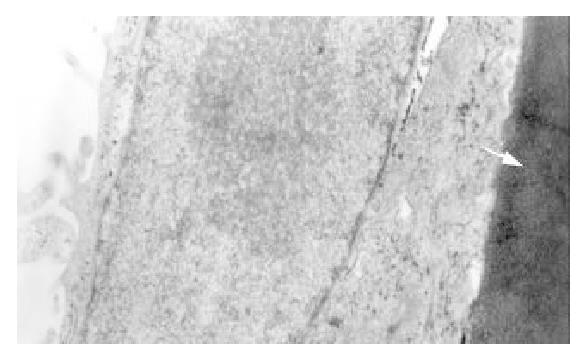
After cultivation with microcarrier for 4 h, all the cells anchored to microcarriers. At the 4th day 70% of microcarriers were filled with cells, at the 6th day all microcarriers’s surface were full of cells or cellular mass (Figure 5), at the 8th day some dead cells could be seen shedded from microcarriers. Under the electronmicroscope, L-02 and L-02/IL-2 cells had normal super-microstructure, such as integral cell membrane, affluent mitochondria, glycogen and rough endoplasmic reticulum (Figure 6); 3) L-02 and L-02/IL-2 began an exponential growth after two days in culture. At the 6th day, the number of alive L-02 was 17.1 ± 0.76 × 106/flask and L-02/IL-2 was 16.8 ± 0.53 × 106/flask. At the day of 10, the number of alive cells were 6.1 ± 0.34 × 106/flask and 5.9 ± 0.52 × 106/flask, respectively.
The trend levels of ALB and UREA of L-02/IL-2 cells were consistent with the cell proliferation. The values reached the peak at the 6th day and decreased with growing cell death. The levels of AST and LDH elevated slowly before the 6th day and rapidly increased with the cell death after the peak of growth. These data had not significantly changed as compared with L-02 cells. However, the levels of IL-2 in IL-2/L-02 cells remarkably exceeded that in L-02 cells in the whole cultural process (P < 0.001) (Table 2).
| Cultivation of day | ||||||
| 0 | 2 | 4 | 6 | 8 | 10 | |
| ALB(mg/L) | ||||||
| L-02 | 0 | 8.58 ± 0.30 | 25.01 ± 0.50 | 53.81 ± 1.64 | 39.13 ± 1.22 | 12.16 ± 0.68 |
| L-02/IL-2 | 0 | 8.23 ± 0.39 | 26.14 ± 0.33 | 52.54 ± 1.28 | 40.42 ± 1.15 | 11.87 ± 0.51 |
| UREA(mmol/L) | ||||||
| L-02 | 0.64 ± 0.03 | 0.90 ± 0.06 | 2.45 ± 0.14 | 5.35 ± 0.13 | 2.92 ± 0.11 | 1.15 ± 0.07 |
| L-02/IL-2 | 0.60 ± 0.05 | 0.85 ± 0.03 | 2.57 ± 0.23 | 5.29 ± 0.17 | 2.93 ± 0.14 | 1.08 ± 0.09 |
| AST(IU/L) | ||||||
| L-02 | 1.0 ± 0.58 | 3.3 ± 0.75 | 14.0 ± 0.58 | 17.6 ± 0.98 | 46.6 ± 1.62 | 70.3 ± 1.80 |
| L-02/IL-2 | 1.0 ± 0.43 | 3.5 ± 0.93 | 15.1 ± 0.65 | 18.6 ± 0.72 | 47.0 ± 1.69 | 72.1 ± 2.04 |
| LDH(IU/L) | ||||||
| L-02 | 15.9 ± 0.69 | 25.3 ± 0.95 | 63.0 ± 1.41 | 87.4 ± 2.07 | 522 ± 11.7 | 687 ± 13.4 |
| L-02/IL-2 | 14.5 ± 0.62 | 25.0 ± 1.05 | 64.6 ± 1.57 | 88.7 ± 2.35 | 534 ± 14.6 | 694 ± 15.7 |
| IL-2 (pg/L) | ||||||
| L-02 | 0 | < 10 | 33.2 ± 2.6 | 78.3 ± 3.5 | 43.6 ± 3.1 | 21.7 ± 1.8 |
| L-02/IL-2 | 0 | 6456 ± 373b | 23765 ± 688b | 52180 ± 1483b | 38643 ± 1104b | 4360 ± 587b |
IL-2, an important regulatory factor in immune network, can induce proliferation of T cell and enhance the immune response function of T cell, B cell, NK cell and monocytes. It plays an important role in antitumor and antiinfection immune function in the body[15-17]. As one of the most therapeutically effective genes, IL-2 gene has been transduced into a varieties of cells in research[8,12,18]. Recently, there were some reports about direct injection of viral vector that expressed IL-2 gene for therapy of hepatocarcinoma[7]. Worldwide, 80% of gene-therapy projects that were applied clinically with approval were using retroviral vector, for example, LNCX, LXCN, LXSN LNSX, etc[19,20]. LNCX, which contained the immediate early promoter of human cytomegalovirus (CMV), was not limited by cell type or animal species and it was more powerful than the other types of enhancer[21]. In our study, LNCX, and its derivation LNCIL-2 were transduced into human hepatocyte line L-02. Clonogeneicity rate of the L-02/IL-2 cells were obviously lower than that of L-02/Neo cells and L-02 cells, due to the change of function of IL-2 as an inhibited signal in cell proliferation. The levels of IL-2 in supernatant of L-02/IL-2 cells were remarkably higher than that of L-02/Neo cells and L-02 cells, and the L-02/IL-2 cells could secrete IL-2 for more than ten weeks. These data showed that IL-2 gene was successfully integrated into the genome of L-02 cells.
Highly differentiated liver cell line were easy to proliferate in culture in vitro, and had many features of normal hepatocyte, so the construction of hepatocyte line is a very significant subject[22]. Japanese scholars had constructed immortalized hepatocyte lines and used them for hepatocyte transplantation and bioartificial liver system with high efficacy[23-26]. In this study, the hepatocyte line we used, L-02, was histologically originated from normal human liver tissue and immortalized. High levels of ALB and UREA reflected the cell’s good biological activity in protein synthesis and amino acid metabolism. When IL-2 gene was inserted, these characteristics were not notably changed.
As a alternative for amplifying the number of cells, the technique of cultivation with microcarrier is increasingly playing an important role in bio-engineering. For example, researchers can use it to produce monoclonal antibody[27], hormone[28], vaccine[29], cytokine[30] and even viral vector for gene therapy[31], and moreover use it to culture hepatocyte for bioartificial liver system[32,33] or hepatocyte transplantation[34,35] for clinical use. Regardless of the type of liver cell, two requirements must be considered: First, the number of cell is adequate and easily available. Second, the cells should form congeries, because the work of hepatocytes depends on the contact between the cells or cell and the matrix. With charges-free, Cytodex3, formed by chemically coupling a thin layer of denatured collagen to the cross-linked dextran matrix, has a good adhesive character. So Cytodex3 became the core of hepatocyte aggregation and gradually formed the matrix-induced liver cell aggregates (MILCA)[36].
The significances of construction of L-02/IL-2 cell are as follow. Theoretically, if hepatocytes modified by IL-2 gene can be transplanted into a patient with HCC who is subjected to operation, these cells might provide some functions of hepatocyte as well as antitumor immune function of IL-2 that can induce the regression of cancer cells and inhibit the metastasis, due to the activation of T-cell and other effector cells. Of course, the immune rejection of transplantation should be taken into account, two strategies are available. (1) The fetal hepatocyes, which are poor in immunogenicity and susceptible to retrovirus[25,37], can be selected to be transfected by target-gene and cultured with microcarrier. (2) Microencapsular technique can be used to encapsule transgenetically immortalized hepatocytes, realizing the continuous expression of exogenous gene[38,39], which can be testified by further animal experimentation.
Edited by Wu XN
| 1. | Strauss M. Liver-directed gene therapy: prospects and problems. Gene Ther. 1994;1:156-164. [PubMed] |
| 2. | Raper SE. Hepatocyte transplantation and gene therapy. Clin Transplant. 1995;9:249-254. [PubMed] |
| 3. | Ojeifo JO, Su N, Ryan US, Verma UN, Mazumder A, Zwiebel JA. Towards endothelial-cell-directed cancer immunotherapy: in vitro expression of human recombinant cytokine genes by human and mouse primary endothelial cells. Cytokines Mol Ther. 1996;2:89-101. [PubMed] |
| 4. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 5. | Tang YC, Li Y, Qian GX. Reduction of tumorigenicity of SMMC-7721 hepatoma cells by vascular endothelial growth factor antisense gene therapy. World J Gastroenterol. 2001;7:22-27. [PubMed] |
| 6. | Wang Z, Qiu SJ, Ye SL, Tang ZY, Xiao X. Combined IL-12 and GM-CSF gene therapy for murine hepatocellular carcinoma. Cancer Gene Ther. 2001;8:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Barajas M, Mazzolini G, Genové G, Bilbao R, Narvaiza I, Schmitz V, Sangro B, Melero I, Qian C, Prieto J. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology. 2001;33:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Hirschowitz EA, Naama HA, Evoy D, Lieberman MD, Daly J, Crystal RG. Regional treatment of hepatic micrometastasis by adenovirus vector-mediated delivery of interleukin-2 and interleukin-12 cDNAs to the hepatic parenchyma. Cancer Gene Ther. 1999;6:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Kim JH, Gong SJ, Yoo NC, Lee H, Shin DH, Uhm HD, Jeong SJ, Cho JY, Rha SY, Kim YS. Effects of interleukin-2 transduction on the human hepatoma cell lines using retroviral vector. Oncol Rep. 1999;6:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Bui LA, Butterfield LH, Kim JY, Ribas A, Seu P, Lau R, Glaspy JA, McBride WH, Economou JS. In vivo therapy of hepatocellular carcinoma with a tumor-specific adenoviral vector expressing interleukin-2. Hum Gene Ther. 1997;8:2173-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Caruso M, Pham-Nguyen K, Kwong YL, Xu B, Kosai KI, Finegold M, Woo SL, Chen SH. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci USA. 1996;93:11302-11306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Huang H, Chen SH, Kosai K, Finegold MJ, Woo SL. Gene therapy for hepatocellular carcinoma: long-term remission of primary and metastatic tumors in mice by interleukin-2 gene therapy in vivo. Gene Ther. 1996;3:980-987. [PubMed] |
| 13. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual, 2nd edition. Cold spring harbor. 1996;55. |
| 14. | Byun J, Kim JM, Kim SH, Yim J, Robbins PD, Kim S. A simple and rapid method for the determination of recombinant retrovirus titer by G418 selection. Gene Ther. 1996;3:1018-1020. [PubMed] |
| 15. | Correale P, Campoccia G, Tsang KY, Micheli L, Cusi MG, Sabatino M, Bruni G, Sestini S, Petrioli R, Pozzessere D. Recruitment of dendritic cells and enhanced antigen-specific immune reactivity in cancer patients treated with hr-GM-CSF (Molgramostim) and hr-IL-2. results from a phase Ib clinical trial. Eur J Cancer. 2001;37:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zheng N, Ye SL, Sun RX, Zhao Y, Tang ZY. Effects of cryopreservation and phenylacetate on biological characters of adherent LAK cells from patients with hepatocellular carcinoma. World J Gastroenterol. 2002;8:233-236. [PubMed] |
| 17. | Chen B, Timiryasova TM, Gridley DS, Andres ML, Dutta-Roy R, Fodor I. Evaluation of cytokine toxicity induced by vaccinia virus-mediated IL-2 and IL-12 antitumour immunotherapy. Cytokine. 2001;15:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Cao XT, Zhang WP, Tao Q. [Enhanced immune functions and antitumor activity of fibroblast-mediated interleukin-2 gene therapy]. Zhonghua Yixue Zazhi. 1995;75:521-524, 573. [PubMed] |
| 19. | Wang X, Liu FK, Li X, Li JS, Xu GX. Inhibitory effect of endostatin expressed by human liver carcinoma SMMC7721 on endothelial cell proliferation in vitro. World J Gastroenterol. 2002;8:253-257. [PubMed] |
| 20. | Fakhrai H, Shawler DL, Van Beveren C, Lin H, Dorigo O, Solomon MJ, Gjerset RA, Smith L, Bartholomew RM, Boggiano CA. Construction and characterization of retroviral vectors for interleukin-2 gene therapy. J Immunother. 1997;20:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Boshart M, Weber F, Jahn G, Dorsch-Häsler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 899] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 22. | Cascio SM. Novel strategies for immortalization of human hepatocytes. Artif Organs. 2001;25:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kobayashi N, Noguchi H, Fujiwara T, Westerman KA, Leboulch P, Tanaka N. Establishment of a highly differentiated immortalized adult human hepatocyte cell line by retroviral gene transfer. Transplant Proc. 2000;32:2368-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Kobayashi N, Miyazaki M, Fukaya K, Inoue Y, Sakaguchi M, Noguchi H, Matsumura T, Watanabe T, Totsugawa T, Tanaka N. Treatment of surgically induced acute liver failure with transplantation of highly differentiated immortalized human hepatocytes. Cell Transplant. 2000;9:733-735. [PubMed] |
| 25. | Kobayashi N, Noguchi H, Watanabe T, Matsumura T, Totsugawa T, Fujiwara T, Tanaka N. Role of immortalized hepatocyte transplantation in acute liver failure. Transplant Proc. 2001;33:645-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Kobayashi N, Noguchi H, Watanabe T, Matsumura T, Totsugawa T, Fujiwara T, Tanaka N. A tightly regulated immortalized human fetal hepatocyte cell line to develop a bioartificial liver. Transplant Proc. 2001;33:1948-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Voigt A, Zintl F. Hybridoma cell growth and anti-neuroblastoma monoclonal antibody production in spinner flasks using a protein-free medium with microcarriers. J Biotechnol. 1999;68:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Hamid M, McCluskey JT, McClenaghan NH, Flatt PR. Culture and function of electrofusion-derived clonal insulin-secreting cells immobilized on solid and macroporous microcarrier beads. Biosci Rep. 2000;20:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Junker BH, Wu F, Wang S, Waterbury J, Hunt G, Hennessey J, Aunins J, Lewis J, Silberklang M, Buckland BC. Evaluation of a microcarrier process for large-scale cultivation of attenuated hepatitis A. Cytotechnology. 1992;9:173-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bing RJ, Dudek R, Kähler J, Narayan KS, Ingram M. Cytokine production from freshly harvested human mononuclear cells attached to plastic beads. Tissue Cell. 1992;24:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Wu SC, Huang GY, Liu JH. Production of retrovirus and adenovirus vectors for gene therapy: a comparative study using microcarrier and stationary cell culture. Biotechnol Prog. 2002;18:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Gao Y, Xu XP, Hu HZ, Yang JZ. Cultivation of human liver cell lines with microcarriers acting as biological materials of bioartificial liver. World J Gastroenterol. 1999;5:221-224. [PubMed] |
| 33. | Suh KS, Lilja H, Kamohara Y, Eguchi S, Arkadopoulos N, Neuman T, Demetriou AA, Rozga J. Bioartificial liver treatment in rats with fulminant hepatic failure: effect on DNA-binding activity of liver-enriched and growth-associated transcription factors. J Surg Res. 1999;85:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Demetriou AA, Levenson SM, Novikoff PM, Novikoff AB, Chowdhury NR, Whiting J, Reisner A, Chowdhury JR. Survival, organization, and function of microcarrier-attached hepatocytes transplanted in rats. Proc Natl Acad Sci USA. 1986;83:7475-7479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Nyberg SL, Peshwa MV, Payne WD, Hu WS, Cerra FB. Evolution of the bioartificial liver: the need for randomized clinical trials. Am J Surg. 1993;166:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Kong LB, Chen S, Demetriou AA, Rozga J. Matrix-induced liver cell aggregates (MILCA) for bioartificial liver use. Int J Artif Organs. 1996;19:72-78. [PubMed] |
| 37. | Koch KS, Brownlee GG, Goss SJ, Martinez-Conde A, Leffert HL. Retroviral vector infection and transplantation in rats of primary fetal rat hepatocytes. J Cell Sci. 1991;99:121-130. [PubMed] |
| 38. | Aoki K, Hakamada K, Umehara Y, Seino K, Itabashi Y, Sasaki M. Intraperitoneal transplantation of microencapsulated xenogeneic hepatocytes in totally hepatectomized rats. Transplant Proc. 2000;32:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Umehara Y, Hakamada K, Seino K, Aoki K, Toyoki Y, Sasaki M. Improved survival and ammonia metabolism by intraperitoneal transplantation of microencapsulated hepatocytes in totally hepatectomized rats. Surgery. 2001;130:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |