Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.73
Revised: August 5, 2002
Accepted: August 16, 2002
Published online: January 15, 2003
AIM: This study investigated the anti-cancer effect of combined quercetin and a recombinant adenovirus vector expressing the human p53, GM-CSF and B7-1 genes (designated BB-102) on human hepatocellular carcinoma (HCC) cell lines in vitro.
METHODS: The sensitivity of HCC cells to anticancer agents was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The viability of cells infected with BB-102 was determined by trypan blue exclusion. The expression levels of human wild-type p53, GM-CSF and B7-1 genes were determined by Western blot, enzyme-linked immunosorbent assay (ELISA) and flow cytometric analysis, respectively. The apoptosis of BB-102-infected or quercetin-treated HCC cells was detected by terminal deoxynucleotidyl transferase (TdT) assay or DNA ladder electrophoresis.
RESULTS: Quercetin was found to suppress proliferation of human HCC cell lines BEL-7402, HuH-7 and HLE, with peak suppression at 50 μmol/L quercetin. BB-102 infection was also found to significantly suppress proliferation of HCC cell lines. The apoptosis of BB-102-infected HCC cells was greater in HLE and HuH-7 cells than in BEL-7402 cells. Quercetin did not affect the expression of the three exogenous genes in BB-102-infected HCC cells (P > 0.05), but it was found to further decrease proliferation and promote apoptosis of BB-102-infected HCC cells.
CONCLUSION: BB-102 and quercetin synergetically suppress HCC cell proliferation and induce HCC cell apoptosis, suggesting a possible use as a combined anti-cancer agent.
- Citation: Shi M, Wang FS, Wu ZZ. Synergetic anticancer effect of combined quercetin and recombinant adenoviral vector expressing human wild-type p53, GM-CSF and B7-1 genes on hepatocellular carcinoma cells in vitro. World J Gastroenterol 2003; 9(1): 73-78
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/73.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.73
Hepatocellular carcinoma (HCC) is one of the most malignant diseases known. Surgical resections are incapable of removing all HCC cells and the disease is very resistant to anticancer agents, making chemotherapy an equally ineffective option[1]. Therefore, anti-tumor gene therapy strategies seem a good future option for the treatment of HCC[2]. Numerous studies have shown that tumor occurrence and progression are often related to mutations in tumor suppressor genes or loss of host anti-tumor immunity[3-9]. Therefore, a promising approach is transfer of tumor suppressor genes to cause tumor cell growth arrest or apoptosis, combined with cytokine genes to induce an effective immune response directly against both the genetically modified and the parental tumor cells[10].
In HCC, mutation or loss of tumor suppressor gene p53 is associated with malignancy and chemoresistance[11-13], making the wild-type p53 (WT-p53) gene a good candidate for replacement by gene therapy. Granulocyte-macrophage colony stimulating factor (GM-CSF) has been proven to be a potent, long-lasting inducer of antitumor immunity that promotes maturation of dendritic cells and enhances the function of antigen-presenting cells and natural killer (NK) cells[14-17]. In addition, the expression of members of the B7 gene family has been shown to be important in antitumor responses in both mice and humans[18-20]. However, most tumor cells, including HCC cells, lack B7-1 molecules on their surface, and as a result they escape recognition by the immune system[21]. Kim et al found an increased antitumor effect with the combined expression of GM-CSF and B7-1 in athymic nude mice, suggesting that coexpression of GM-CSF and B7-1 may enhance antitumor activity[22]. Recombinant adenovirus vector BB-102, which expresses p53, GM-CSF and B7-1 genes[23], has been shown to inhibit the growth of various human carcinoma cells, such as hepatocellular carcinoma, lung cancer[24], and laryngeal cancer[25], and enhances carcinoma cell chemosensivity to anti-cancer agents.
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is one of most widely distributed bioflavonoids in the plant kingdom and is a common component of most edible fruits and vegetable. Humans consume approximately 1 g of dietary flavonoid daily. This compound has been shown to inhibit the growth of various human cancer cell lines[26-34], including leukemia, hepatocellular carcinoma, and estrogen-receptor positive breast carcinoma MCF-7, suggesting that quercetin may have anti-cancer and anti-metastasis potential[35-39]. The growth inhibitory effect of quercetin on tumor cells is found to be consequence of its ability to interfere with the enzymatic processes involved in the regulation of cellular proliferation: DNA, RNA and protein biosynthesis[40-43]. Quercetin also has shown to down-regulate or inhibit the phosphatidylinositol (PI) and phosphatidylinositol phosphate (PIP) kinase activities in human carcinoma cells, leading to a marked reduction of second messengers IP3 concentration and cell death[44,45]. Therefore, quercetin may be useful in the treatment of carcinomas with increased or down-regulated signal transduction capacity[45-47].
These results suggest that the combination of quercetin with BB-102 may synergetically suppress the growth of carcinoma cells. Accordingly, this study addressed the synergetic action of quercetin combined with BB-102 against HCC cells in vitro.
Quercetin was purchased from Sigma (St Louis, MO). Western blot detection system ECL + plus Kit was purchased from Amersham Pharmacia Biotech (Arlinton Heights, IL). GM-CSF ELISA kit was bought from Genzyme (Cambridge, MA). The TdT FragELTM DNA fragmentation detection kit was from Calbiochem (Cambridge, MA). Ad-GFP (Ad5 vector expressing green fluorescence protein) was kindly provided by the Gene Therapy Unit, Baxter Healthcare Corporation, USA. BB-102 was reconstructed by Dr ZH Qu. Quercetin was dissolved in medium containing 0.1% dimethyl sulfoxide (DMSO), and the same concentration of DMSO was used in control experiments.
The BEL-7402, HLE and HuH-7 cell lines were seeded in 96-well plates at a density of 5 × 103 cells/well. After 24 h, the cells were exposed to various concentrations of quercetin from 3.125 μmol/L to 100 μmol/L. After 72 h, the number of viable cells was determined by MTT assay.
HCC cell lines were seeded in six-well plates at a density of 5 × 105 cells/well. Twelve hours later, the cells were infected with BB-102 and Ad-GFP at a MOI of 50 pfu/cell. Culture medium was used for mock infection. Triplicate wells of each treatment group were counted every 2 days for a total of four times after infection. The viability of the cells was determined by trypan blue exclusion.
Apoptosis induced by BB-102 was analyzed by terminal deoxynucleotidyl transferase (TdT) assay. Briefly, HCC cells were seeded in 12-well plates at a density of 5 × 10 cells/well. After 24 h, they were infected with BB-102 or Ad-GFP at a MOI of 50 pfu/cell. Culture medium was used for mock infection. Seventy-two hours later, cell monolayers were fixed with 4% formaldehyde diluted in phosphate-buffered saline (PBS) for 15 min at room temperature. Apoptosis of the cells was detected using a TdT FragELTM DNA fragmentation detection kit according to the protocol.
Cellular apoptosis induced by quercetin or quercetin combined with BB-102 was analyzed by agarose gel-electrophoresis as described based on the pattern of DNA cleavage. Briefly, cells (1 × 106) were lysed with 0.5 ml lysis buffer, followed by the addition of RNase A to a final concentration of 200 μg/ml, and incubated for 1 h at 37 °C. Cells were then treated with 300 μg/ml of proteinase K for 1 h at 37 °C. After addition of 4 μl loading buffer, 20 μl samples in each lane were subjected to electrophoresis on a 1.5% agarose at 50 V for 3 h. DNA was stained with ethidium bromide and laddering was visualized under UV light.
Three cell lines were seeded in 6-well plates at a density of 1 × 105 cells/well. Group II was treated with 100 μmol/L quercetin, group III was infected with BB-102 at a MOI of 50 pfu/cell, group IV was infected with BB-102 and then treated with 100 μmol/L quercetin, and group I was mock-infected with culture medium infected with empty adenovirus vector as control. After 48 h incubation, cells were washed with phosphate buffered saline, disrupted by addition of lysing buffer (100 mmol/L Tris·Cl pH6.8, 200 mmol/L DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerol), and electrophoresed. Western blot detection system ECL + plus Kit was used to determine p53 expression according to the instruction manual.
Expression of human GM-GSF was detected by enzyme-linked immunosorbent assay. Briefly, the cells were seeded in 6-well plates at a density of 1 × 106 cells/well. After 24 h, the cells were infected with BB-102 at a MOI of 50 pfu/cell for 1 h, then BB-102 suspensions were replaced with either culture medium alone, or culture medium containing 100 μmol/L quercetin. After 48 h, the suspension was collected. The GM-CSF present in each suspension was quantified using the ELISA kit according to the protocol.
HCC cells were seeded in 6-well plates at a density of 5 × 105 cells/well. After 24 h, the cells were either treated with 100 μmol/L quercetin, infected with BB-102 at a MOI of 50 pfu/cell, or infected with BB-102 at a MOI of 50 pfu/cell then treated with 100 μmol/L quercetin. Culture medium was used for mock infection. After 48 h incubation, the cells were collected. Cells were washed twice in PBS and resuspended in PBS containing 1% bovine serum albumin (BSA) prior to incubation with anti-human B7-1 monoclonal antibody (MoAb) for 30 min at 4 °C in the dark, followed by two washes in PBS/BSA. The cells were stained with FITC-conjugated goat anti-mouse IgG for 30 min at 4 °C. Nonspecific binding was controlled by incubation with isotypic controls. Fluorescence was measured with FACSCalibur flow cytometer (Becton Dickinson).
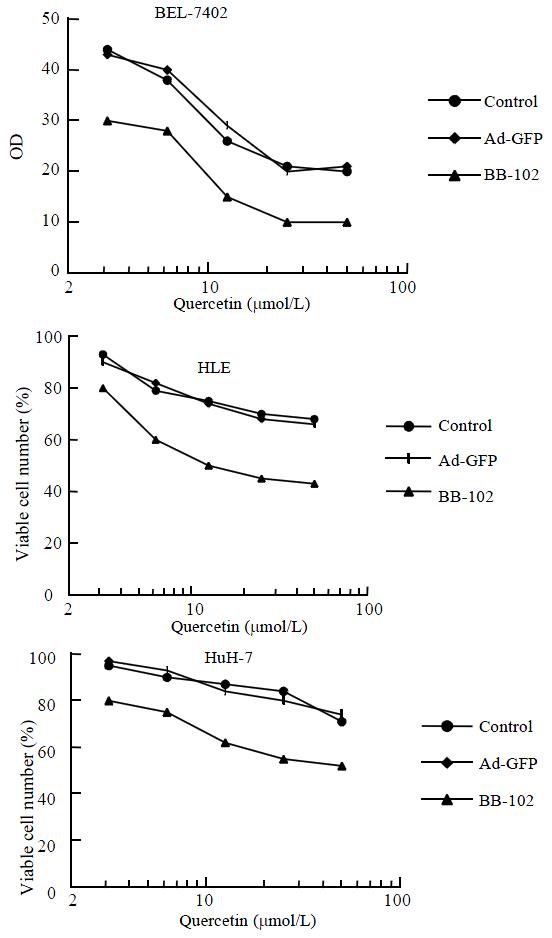
HCC cells were seeded in 96-well plates at a density of 5 × 103 cells/well. After 24 h, cells were infected with Ad-GFP or BB-102 at a MOI of 50 pfu/cell. Culture medium was used for mock infection. After 48 h, cells were treated with various concentrations of quercetin from 3.125 to 50 μmol/L. Culture medium was used for mock treatment. After 72 h, the viable cell numbers were tested by MTT assay.
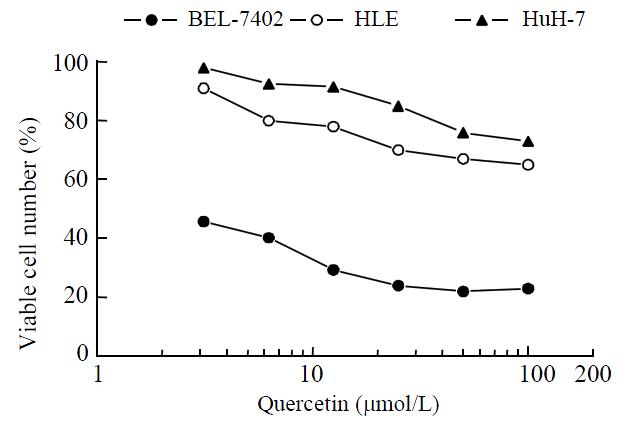
Quercetin was shown to inhibit HCC proliferation and induce HCC apoptosis in vitro in a dose-dependent manner until it reached peak inhibition at 50 μmol·L-1 (Figure 1).
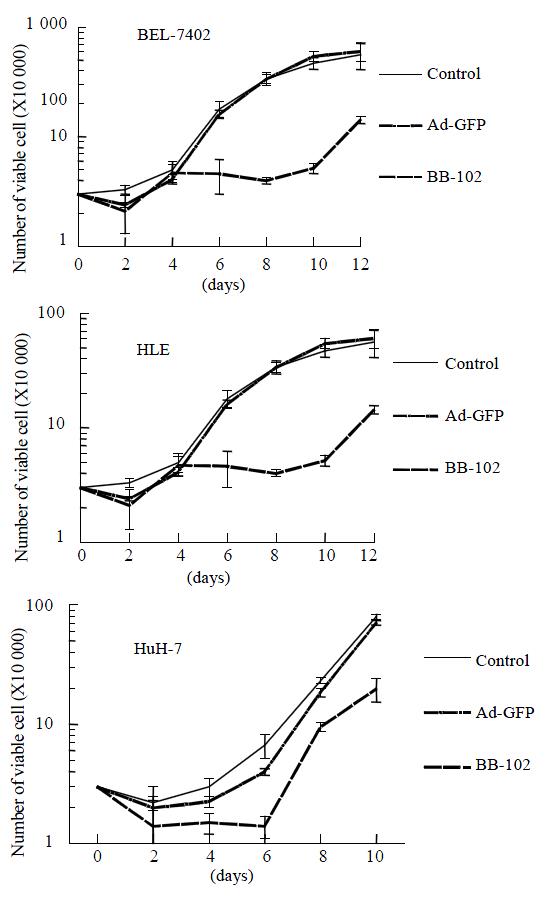
Introduction of p53, GM-CSF and B7-1 through BB-102 infection led to significant suppression of growth proliferation in BEL-7402, HLE and HuH-7 cells compared with those infected with Ad-GFP or mock infected (Figure 2). Among them, there was a lower suppression in BEL-7402 cell line compared with HLE and HuH-7 cell lines (P < 0.05), which might be related to the fact that BEL-7402 expresses an endogenous wild-type p53, while HLE and HuH-7 both express endogenous mutant p53, resulting in BEL-7402 being less sensitive to the effects of the exogenous p53 protein expressed by BB-102.
As measured by TdT assay, apoptotic rates in BEL-7402, HLE and HuH-7 cells lines were were 12.75%, 57% and 49.5%, respectively. This suggests that HLE and HuH-7 cells were more sensitive to BB-102 than BEL-7402 cells.
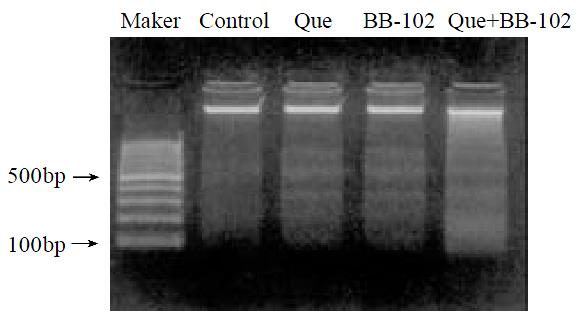
DNA laddering (Figure 3) suggests that quercetin induces HCC cell apoptosis, and that BB-102 further promoted the quercetin-treated cell apoptosis.
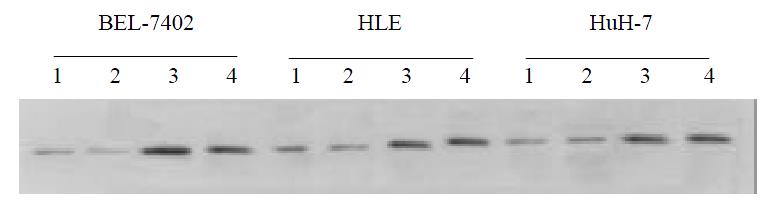
Expression of exogenous P53 in BB-102-infected cells By using Western blot analysis, high levels of p53 protein were detected in BB-102-infected HCC cell lines. These levels were not affected by treatment with quercetin (Figure 4).
Expression of exogenous GM-CSF protein in BB-102-infected cells ELISA assay showed that a high-level expression of GM-CSF was detected in the culture suspension of BB-102-infected cells 48 h after infection. There was no statistical difference in GM-CSF level between the cells treated with quercetin and untreated cells (Table 1).
| Cell line | Infected with BB-102 | Infected with BB-102+Que |
| BEL-7402 | 118.9 ± 29.9 | 147.7 ± 13.2 |
| HLE | 209.6 ± 55.7 | 248.3 ± 15.9 |
| HuH-7 | 250.7 ± 21.9 | 283.7 ± 21.6 |
Expression of exogenous B7-1 protein in BB-102-infected cells B7-1 gene expression was observed flow cytometric analysis 48 h after BB-102 infection (Table 2). There was no significant difference in B7-1 expression between cells treated with quercetin and untreated cells.
The proliferation suppression pattern of HCC cells treated with quercetin was not significantly different between Ad-GFP-infected cells and Ad-mock infected cells. In contrast, BB-102-infected cells showed a significant increase of suppression when treated with quercetin. This implies that BB-102 transduction and quercetin treatment synergistically suppress HCC proliferation (Figure 5). The control data indicates that it was the exogenous gene expression that induced this effect, not the adenovirus.
Gene therapy, the introduction of functional genes into cells to treat or prevent a disease, is a promising approach for the treatment of human cancer. In an effort to explore potential gene therapy approaches to the treatment of stubborn HCC, we investigated the synergetic effects of dietary component (quercetin) plus gene transfer (BB-102 infection). BB-102 is a recombinant adenoviral vector expressing the genes for human wild-type p53, GM-CSF and B7-1[23]. Our study showed that BB-102 infection arrested the growth of HCC cells lines BEL-7402, HLE, HuH-7, and induced their apoptosis by the expression of exogenous wild-type p53 (a transcriptional activator that induces cell cycle arrest and apoptosis[48-50]). BB-102 also enhanced immunogenicity of HCC cells and improved host antitumor immune reaction.
We then examined the possible synergy between these exogenous genes and quercetin, which is thought to have a long-term preventive effect on chemical carcinogenesis, especially in people who eat a diet rich in fruits and vegetables[30,31,36,51]. Previously, we had found that antineoplastic drug concentrations exerting cytotoxic activity were markedly lower when cells were pretreated with quercetin[28,35]. In addition, quercetin has been shown to inhibit the growth of human breast cancer cell line MDA-MB468 in a dose-dependent fashion by specifically inhibiting the expression of mutant p53 in cellular transformation[52]. Because HCC patients are generally resistant to chemotherapy (possibly due to the loss of a functional p53 gene[53], which is known to occur in 50% of cancers[54-57]), we questioned whether quercetin could reverse the multi-drug resistance of HCC cells, perhaps through down-regulation of mutated p53 or glycoprotein[52,58,59]. In addition to its possible role in adjusting p53 levels, quercetin is also known to inhibit the synthesis of HSP70 (heat shock protein 70) and change its intracellular distribution. HSP70 is involved in apoptosis, and the link between the two suggests that quercetin may be involved in the induction of apoptosis[35,60].
We found enhancement of apoptosis and inhibition of the proliferation of BB-102-infected HCC cell lines treated with quercetin, suggesting that there is a synergetic anticancer effect between quercetin and BB-102. There was no change in p53, GM-CSF and B7-1 gene expressions in BB-102-infected HCC cell lines following quercetin treatment, indicating that quercetin did not affect exogenous gene expression in BB-102-infected HCC cells. We believe it is possible that this synergetic effect might be due to BB-102 transduction promoting the chemosensitivity of HCC cells to quercetin, but this and other mechanisms will need further study.
BEL-7402 cells were more sensitive to quercetin than HLE and HuH-7 cells. At a concentration of 50 umol/L quercetin (peak proliferation inhibition), proliferation inhibition was 78%, 33% and 24% for BEL-7402, HLE and HuH-7, respectively. Our previous studies have shown that the doubling time of BEL-7402 is the shortest among these cells. This suggests that the cells that proliferate quickly are more sensitive to quercetin in this study. In addition, quercetin induced apoptosis in HCC cells at G1 and S in a dose- and time-dependent manner, and the effect was enhanced by BB-102 infection. This enhancement was less in BEL-7402, possibly due to its wild-type endogenous p53 status[61]. Overall, HCC cells treated with both quercetin and BB-102 showed inhibition of proliferation and increased apoptosis, suggesting that quercetin may be useful as an adjuvant in chemotherapy treatment of HCC patients. The combination of quercetin and BB-102 transduction is a promising new strategy for the treatment of typically chemo-resistant hepatomas.
Edited by Wu XN
| 1. | Venook AP. Hepatocellular carcinoma. Curr Treat Options Oncol. 2000;1:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Mohr L, Geissler M, Blum HE. Gene therapy for malignant liver disease. Expert Opin Biol Ther. 2002;2:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Peller S. Clinical implications of p53: effect on prognosis, tumor progression and chemotherapy response. Semin Cancer Biol. 1998;8:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Friess H, Kleeff J, Korc M, Büchler MW. Molecular aspects of pancreatic cancer and future perspectives. Dig Surg. 1999;16:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 324] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Canote R, Du Y, Carling T, Tian F, Peng Z, Huang S. The tumor suppressor gene RIZ in cancer gene therapy (review). Oncol Rep. 2002;9:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Farid NR. P53 mutations in thyroid carcinoma: tidings from an old foe. J Endocrinol Invest. 2001;24:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Müschen M, Warskulat U, Beckmann MW. Defining CD95 as a tumor suppressor gene. J Mol Med (Berl). 2000;78:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Wu Q, Moyana T, Xiang J. Cancer gene therapy by adenovirus-mediated gene transfer. Curr Gene Ther. 2001;1:101-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Xu GW, Sun ZT, Forrester K, Wang XW, Coursen J, Harris CC. Tissue-specific growth suppression and chemosensitivity promotion in human hepatocellular carcinoma cells by retroviral-mediated transfer of the wild-type p53 gene. Hepatology. 1996;24:1264-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] |
| 13. | Buendia MA. Genetics of hepatocellular carcinoma. Semin Cancer Biol. 2000;10:185-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Yu JS, Burwick JA, Dranoff G, Breakefield XO. Gene therapy for metastatic brain tumors by vaccination with granulocyte-macrophage colony-stimulating factor-transduced tumor cells. Hum Gene Ther. 1997;8:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Hogge GS, Burkholder JK, Culp J, Albertini MR, Dubielzig RR, Keller ET, Yang NS, MacEwen EG. Development of human granulocyte-macrophage colony-stimulating factor-transfected tumor cell vaccines for the treatment of spontaneous canine cancer. Hum Gene Ther. 1998;9:1851-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Lu L, Hsieh M, Oriss TB, Morel PA, Starzl TE, Rao AS, Thomson AW. Generation of DC from mouse spleen cell cultures in response to GM-CSF: immunophenotypic and functional analyses. Immunology. 1995;84:127-134. [PubMed] |
| 17. | Ogawa T, Kusumoto M, Mizumoto K, Sato N, Tanaka M. Adenoviral GM-CSF Gene Transduction into Breast Cancer Cells Induced Long-Lasting Antitumor Immunity in Mice. Breast Cancer. 1999;6:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Felzmann T, Ramsey WJ, Blaese RM. Anti-tumor immunity generated by tumor cells engineered to express B7-1 via retroviral or adenoviral gene transfer. Cancer Lett. 1999;135:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Hörig H, Lee DS, Conkright W, Divito J, Hasson H, LaMare M, Rivera A, Park D, Tine J, Guito K. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Takahashi T, Hirano N, Takahashi T, Chiba S, Yazaki Y, Hirai H. Immunogene therapy against mouse leukemia using B7 molecules. Cancer Gene Ther. 2000;7:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Nakatsuka K, Sugiyama H, Nakagawa Y, Takahashi H. Purifi-cation of antigenic peptide from murine hepatoma cells recog-nized by class-I major histocompatibility complex molecule-restricted cytotoxic T-lymphocyte induced with B7-1-gene-trans-fected hepatoma cells. J Hepatol. 1999;30:1119-1129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kim KY, Kang MA, Nam MJ. Enhancement of natural killer cell-mediated cytotoxicity by coexpression of GM-CSF/B70 in hepatoma. Cancer Lett. 2001;166:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Qiu ZH, Lao MF, WU ZZ. Construction of recombinant aden-ovirus co-expressing human wild-type p53, GM-CSF and B7-1genes. Zhongguo Shengwuhuaxue Yu Fengzishengwu Xuebao. 2001;17:33-38. |
| 24. | Qiu ZH, Lao MF, Wang YF, Wu ZZ. Adenovirus mediate multigenes expressing in lung cancer and inducing apoptosis. Zhongguo Zhongliu Shengwuzhiliao Zazhi. 1999;6:83-86. |
| 25. | Qiu Z, Lao M, Wu C. Co-transfer of human wild-type p53 and granulocyte-macrophage colony-stimulating factor genes via recombinant adenovirus induces apoptosis and enhances immunogenicity in laryngeal cancer cells. Cancer Lett. 2001;167:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Larocca LM, Teofili L, Sica S, Piantelli M, Maggiano N, Leone G, Ranelletti FO. Quercetin inhibits the growth of leukemic progenitors and induces the expression of transforming growth factor-beta 1 in these cells. Blood. 1995;85:3654-3661. [PubMed] |
| 27. | Scambia G, Panici PB, Ranelletti FO, Ferrandina G, De Vincenzo R, Piantelli M, Masciullo V, Bonanno G, Isola G, Mancuso S. Quercetin enhances transforming growth factor beta 1 secretion by human ovarian cancer cells. Int J Cancer. 1994;57:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ranelletti FO, Ricci R, Larocca LM, Maggiano N, Capelli A, Scambia G, Benedetti-Panici P, Mancuso S, Rumi C, Piantelli M. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int J Cancer. 1992;50:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Pawlikowska-Pawlega B, Jakubowicz-Gil J, Rzymowska J, Gawron A. The effect of quercetin on apoptosis and necrosis induction in human colon adenocarcinoma cell line LS180. Folia Histochem Cytobiol. 2001;39:217-218. [PubMed] |
| 31. | Kampa M, Hatzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer. 2000;37:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Iwashita K, Kobori M, Yamaki K, Tsushida T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 2000;64:1813-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Uddin S, Choudhry MA. Quercetin, a bioflavonoid, inhibits the DNA synthesis of human leukemia cells. Biochem Mol Biol Int. 1995;36:545-550. [PubMed] |
| 34. | Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Sliutz G, Karlseder J, Tempfer C, Orel L, Holzer G, Simon MM. Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: implication of quercetin as sensitiser in chemotherapy. Br J Cancer. 1996;74:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 779] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 37. | Wang HK. The therapeutic potential of flavonoids. Expert Opin Investig Drugs. 2000;9:2103-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Asaum J, Matsuzaki H, Kawasak S, Kuroda M, Takeda Y, Kishi K, Hiraki Y. Effects of quercetin on the cell growth and the intracellular accumulation and retention of adriamycin. Anticancer Res. 2000;20:2477-2483. [PubMed] |
| 39. | Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Yamashita N, Kawanishi S. Distinct mechanisms of DNA damage in apoptosis induced by quercetin and luteolin. Free Radic Res. 2000;33:623-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Musonda CA, Helsby N, Chipman JK. Effects of quercetin on drug metabolizing enzymes and oxidation of 2',7-dichlorofluorescin in HepG2 cells. Hum Exp Toxicol. 1997;16:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Exon JH, Magnuson BA, South EH, Hendrix K. Dietary quercetin, immune functions and colonic carcinogenesis in rats. Immunopharmacol Immunotoxicol. 1998;20:173-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Drewa G, Woźqak A, Pałgan K, Schachtschabel DO, Grzanka A, Sujkowska R. Influence of quercetin on B16 melanotic melanoma growth in C57BL/6 mice and on activity of some acid hydrolases in melanoma tissue. Neoplasma. 2001;48:12-18. [PubMed] |
| 44. | Weber G, Shen F, Yang H, Prajda N, Li W. Regulation of signal transduction activity in normal and cancer cells. Anticancer Res. 1999;19:3703-3709. [PubMed] |
| 45. | Weber G, Shen F, Prajda N, Yeh YA, Yang H, Herenyiova M, Look KY. Increased signal transduction activity and down-regulation in human cancer cells. Anticancer Res. 1996;16:3271-3282. [PubMed] |
| 46. | Richter M, Ebermann R, Marian B. Quercetin-induced apoptosis in colorectal tumor cells: possible role of EGF receptor signaling. Nutr Cancer. 1999;34:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Shen F, Herenyiova M, Weber G. Synergistic down-regulation of signal transduction and cytotoxicity by tiazofurin and quercetin in human ovarian carcinoma cells. Life Sci. 1999;64:1869-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5482] [Cited by in RCA: 5459] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 49. | Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855-4878. [PubMed] |
| 50. | Hui AM, Makuuchi M, Li X. Cell cycle regulators and human hepatocarcinogenesis. Hepatogastroenterology. 1998;45:1635-1642. [PubMed] |
| 51. | Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999;31 Suppl:S75-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994;54:2424-2428. [PubMed] |
| 53. | Thottassery JV, Zambetti GP, Arimori K, Schuetz EG, Schuetz JD. p53-dependent regulation of MDR1 gene expression causes selective resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 1997;94:11037-11042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Wang D, Shi JQ. Overexpression and mutations of tumor sup-pressor gene p53 in hepatocellular carcinoma. China Natl J New Gastroenterol. 1996;2:161-164. |
| 55. | Wang XJ, Yuan SL, Li CP, Iida N, Oda H, Aiso S, Ishikawa T. Infrequent p53 gene mutation and expression of the cardia adenocarcinomas from a high-incidence area of Southwest China. World J Gastroenterol. 2000;6:750-753. [PubMed] |
| 56. | Li ZX, Liu PY, Xu WX, Cong B, Ma ZX, Li Y. p53 gene mutations in primary gastric cancer. China Natl J New Gastroenterol. 1996;2:41-43. |
| 57. | Peng XM, Peng WW, Yao JL. Codon 249 mutations of p53 gene in development of hepatocellular carcinoma. World J Gastroenterol. 1998;4:125-127. [PubMed] |
| 58. | Scambia G, Ranelletti FO, Panici PB, De Vincenzo R, Bonanno G, Ferrandina G, Piantelli M, Bussa S, Rumi C, Cianfriglia M. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol. 1994;34:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Beniston RG, Morgan IM, O'Brien V, Campo MS. Quercetin, E7 and p53 in papillomavirus oncogenic cell transformation. Carcinogenesis. 2001;22:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Wei YQ, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of apoptosis by quercetin: involvement of heat shock protein. Cancer Res. 1994;54:4952-4957. [PubMed] |
| 61. | Shi M, Wang FS, Liu MX, Jin L, Shi H, Lei ZY. The examination of p53 gene status in BEL-7402, HLE and HuH-7cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1330-1332. |