Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.961
Revised: August 12, 2002
Accepted: August 20, 2002
Published online: December 15, 2002
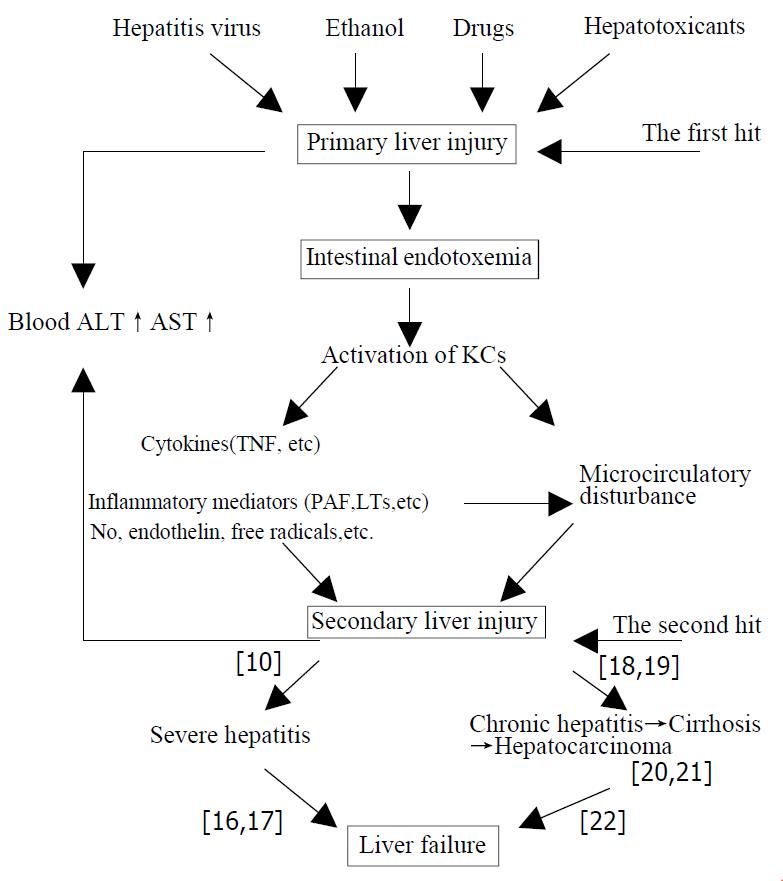
Liver injury induced by various pathogenic factors (such as hepatitis virus, ethanol, drugs and hepatotoxicants, etc.) through their respective special pathogenesis is referred to as “primary liver injury” (PLI). Liver injury resulted from endotoxin (lipopolysaccharide, LPS) and the activation of Kupffer cells by LPS while intestinal endotoxemia (IETM) occurred during the occurrence and development of hepatitis is named the “secondary liver injury” (SLI).The latter which has lost their own specificities of primary pathogenic factors is ascribed to IETM. The “secondary liver injury” is of important action and impact on development and prognosis of hepatitis. More severe IETM commonly results in excessive inflammatory responses, with serious hepatic necrosis, further severe hepatitis and even induces acute liver failure. The milder IETM successively precipitates a cascade, including repeated and persistent hepatocytic impairment accompanied by infiltration of inflammatory cells, hepatic fibrosis, cirrhosis and hepatocarcinoma. Generally, the milder IETM ends with chronic hepatic failure. If PLI caused by various pathogenic factors through their independent specific mechanismis regarded as “the first hit” on liver, then SLI mediated by different chemical mediators from KCs activated by IETM in the course of hepatitis is “the second hit” on liver. Thus, fusing and overlapping of the primary and scondary liver injuries determine and influeuce the complexity of the illness and outcome of the patient with hepatitis. For this reason, the viewpoint of “SLI” induced by the “second hit” on liver inflicted by IETM suggests that medical professionals should attach great importance to both “PLI” and “SLI” caused by IETM. That is, try to adjust the function of KSs and eliminate endotoxemia of the patient.
- Citation: Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol 2002; 8(6): 961-965
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/961.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.961
Gut is a vast pool of bacteria and endotoxins (lipopolysaccharide, LPS) in the body. There are endotoxemia in severe trauma, burn or scald, Intestinal ischemia and liver diseases. They are mainly resulted from large quantities of endotoxin produced by overgrowth of gram-negative bacteria in gut that are increasingly taken into portal vein because of the incereased permeability of intestinal wall. Endotoxemia will be generated if the level of endotoxin surpasses the hepatic capacity for endotoxin scavenging due to decreased phagocytic ability of liver kupffer cells (KCs) and then endotoxin spills over into systemic circulation. For these reasons, it is named IETM because endotoxin comes from gut.
In recent years, the relationship between IETM and liver diseases has been increasingly paid much attention to. Animal studies have demonstrated that various experimental liver diseases were commonly accompanied by IETM. Clinical observations have also showed evidences that the highest incidence(80%-100%) and severe degree of IETM in patients with severe hepatitis was generally recognized, though the incidences of IETM in patients with acute or chronic hepatitis, severe hepatitis, cirrhosis and hepatocarcinoma were not reported equally. Incidences of IETM investigated in our institute in acute or chronic hepatitis, severe hepatitis and cirrhosis were 75%, 79%, 93.3% and 84.3% respectively[1].
LPS activates KCs via two pathways: one is membrane attachment from mCD14 which is the classical CD14 dependent pathway, that requires LPS binding protein (LBP) as a cofactor carring LPS to the membrane of KCs bound to the receptor CD14 ; the soluble compound formed by combining LBP with LPS increases affinity of LPS with CD14. Whereas the other pathway-sCD14 may not require LBP, combines with corresponding receptor on KCs by aid of other proteins (such as HDL, LDL etc). These two pathways finally activate signal transduction system and trigger the synthesis and release of cytokines and inflammatory mediators[2].
KCs stimulated by LPS release chemostatic substances such as TNF-α, interleukins, leukotriene B4 and complement C5, which attract circulating neutrophilic leucocytes. Activated neutrophilic leucocytes up-regulate adhesion molecules receptor (CD11b/CD18) on its membrane surface, then adhere to endothelial cells of hepatic sinusoids. In the meanwhile, adhesion molecules ICAM-1 and ELAM-1 on surface of endothelial cells are also up-regulated and precipitate neutrophilic leucocytes moving to hepatic sinusoids. Oxygen-derived free radicals produced by these activated neutrophilic leucocytes cause lipid peroxidation of cells. Moreover, toxic mediators (PAF, NO, ET-1 etc) released from KCs enhance further liver injury and make platelets aggregated into microthrombi. Hepatic sinusoids being blocked by adhering neutrophilic leucocytes, Platelets and swelling KCs induce slowing of blood flow with following hypoxia. The onset of liver necrosis in this situation is ascribed mainly to abnormal microcirculation. Therefore, liver damage is an overall outcome that is induced by the interactions between inherent KCs, immigranting neutrophilic leucocytes and hepatocytes[3].
Additionally, it shoud be mentioned that LPS not only induces liver necrosis directly or indirectly by chemical mediators released from activated KDs, but also is a promoting factor for other hepatotoxicants (such as D-galactosamine, tetrachloride [CCl4],ethanol, etc) to induce liver necrosis. The use of anti-LPS antibody can significantly attenuate liver necrosis induced by hepatotoxicants and prevent from the occurrence of acute liver failure[4].
There are still some arguments on whether or not LPS directly injures hepatocytes at present. It is reported that there is high content of lipoid (phospholipid mainly) in the membrane of hepatocellular mitochondria. The affinity of diphosphatidyl glycerol, which is the special component of the lipoid, with is the special component of the lipoid, with LPS is very strong. Its acyl group that binds to LPS induces the structural damage of mitochondria, interferes with biological oxidation, inhibits production of ATP and causes hepatocellular injury. LPS can directly insert into double layers of lipid molecule of membrane or conjugate to membrane receptors and initiate the metabolism of membrane phosphatidyl inositol. Large quantities of phosphatidic acid (PA) and inositol triphosphate (IP3) are produced. Degeneration and necrosis of hepatocytes are induced by elevated Ca2+ in cytoplasm, which is resulted from PA with Ca2+ carrier-like action and IP3 which causes the opening of Ca2+ channel in membrane, with great deal of Ca2+ flowing the cell. Structural and functional injuries of hepatocytes and mitochondria can also be caused by degradation of membrane phospholipid and lipid peroxidation mediated by PLA2 activation induced by LPS. LPS phagocytosed by KCs can damage the membrane of lysosome and lead to release of various lysosomal enzymes resulting in cell autolysis.
To explore LPS directly acting on hepatocytes in vitro, we found that hepatocytes showed typical ladder pattern of apoptotic cells morphologically and this process could be inhibited by apoptotic inhibitor ATA. The number of apoptotic hepatocytes was proportional with the dosage of LPS; the number of apoptotic hepatocytes treated with LPS within 24 hours was also in positive ratio with time. It implicated that LPS could directly induce apoptosis of hepatocytes and this action was more prominent at the early phase of treatment[5].
The interaction of LPS with KCs plays a determinant role during the occurance of liver injury and acute liver failure. It was confirmed in a large number of animal experiments that liver injury induced by CCl4, D-galactosamine, thioacetamide (TAA), ethanol, etc. could be reduced or prevented by performing colectomy or administering antibiotics (such as polymyxin B, mycifradin) to decrease the level of intestinal LPS, or by administering gadolinium chloride (GdCl3), silica (SiO2)to block KCs.
The research performed by Ahmad et al[6] indicated that there was high expression of ICAM-1 on hepatocytes, KCs, endothelial cells during acute endotoxemia; there was no expression of ICAM-1 on hepatocytes and KCs when KCs were blocked by GdCl3; there is no occurrence of TNF-α and LTB4, which is released from KCs activated by LPS, attracting neutrophilic leucocytes to infiltrtate into liver. These results implicated that KCs played a vital role in hepatitis and liver injury induced by LPS.
Many researches suggested that ethanol can increase permeability of intestinal mucosa, macromolecules such as LPS were intaken into blood and the plasma level of LPS thus rose up. While phagocytosing LPS, KCs released chemical mediators such as TNF-α, PGE2 to promot oxidation of alcohol in the liver. Owing to increased oxygen consumpsion, hepatocytes was injured by free radicals formed in the state of hypoxia. Experiments verified that the level of plasma LPS in alcoholism animals was significantly correlated with hepatic pathological change (cellular steatosis and necrosis, inflammatory infiltration); the steatosis and necrosis of liver induced by alcohol could be prevented by administering GdCl3 to block KCs[7].
D-galactosamine can induce focal necrosis of whole hepatic lobule with severe infiltration of neutrophlic leucocytes, but liver injury can be thoroughly prevented by GdCl3 injection in caudal vein before D-galactosamine injection intraperitoneally in rats. Liver injury and apoptosis induced by D-galactosamine and small dose of LPS can be markedly alleviated via treatment with TNF-α antiserum, so did alcoholic liver injury. Because TNF-α was mainly stemmed from activated KCs, the key role of KCs played in the liver injury was thus certified[8,9].
In summary, previous researches on mechanism of liver injury were mostly confined to parenchymal liver cells, whereas large amount of current experiments have demonstrated that activation of KC by LPS plays a more important role in the occurance and development of liver injury.
Based upon the above-mentioned understanding we have conceived the following hypothesis on liver injury induced by various pathogenic factors and the development and prognosis of hepatitis (Figure 1)
The “primary liver injury (PLI)” is the hepatic damage that various pathogenic factors (such as hepatitis virus, alcohol, drugs, hepatotoxicants, etc.)induced by specific mechanism separately. In contrast, IETM is formed in the course of the development of hepatitis. Liver injury, which is resulted from LPS, activation of KCs by LPS and others, is refered to as “secondary liver injury (SLI)” The SLI which has lost their own specificities of primary pathogenic factors is ascribed to IETM.
The SLI has an important effect on the development and prognosis of various hepatitis. The severe IETM, which commonly causes over-inflammatory reaction and serious hepatic necrosis, will lead to severe hepatitis and even acute hepatic failure[10]. The mild IETM will successively precipitate a cascade, including the repeated and persistent hepatocellular injury accompanied by infiltration of in- flammatory cells, hepatic fibrosis, cirrhosis and even hepatocarcinoma. Generally, it ends with chronic hepatic failure[11].
Certainly, the mechanism of occurrence and development of various viral hepatitis is not so simple but a considerably complicated issue related to virus itself, host immune state, etc. It is difficult to cover it by only one hypothesis.
Hepatic damage induced by various pathogenic factors (such as hepatitis virus, alcohol, drugs, hepatotoxicants,etc) via specific mechanism separately is named the “primary liver injury (PLI)”.
Hepatic damage resulted from various hepatitis virus is mainly ascribed to host immune reaction. Cytotoxic T lymphocytes (CTLs)attack hepatocytes with HbcAg, HLA-1 and ICAM-1. It results in lysis and apoptosis of a large amount on of hepatocytes. Drugs cause hepatic damage either by direct action, metabolic product or immune mechanism. Ethanol may give rise to be oxidative stress and direct necrosis of hepatocytes in alcoholic liver injury. The new antigen formed by the combination of lipid peroxide products, acetaldehyde (ethanol-derived metabolite) and protein triggers immunity reaction and makes CTLs to attack hepatocytes. TAA, a hepatotoxicant, is taken by liver then is metabolized into TAA-sulfoxide by cytochrome P450 mixed-function oxidase and further transformed into the intermediates and other polar molecules, which irreversibly combine with intrahepatic biomacromolecules to cause hepatic necrosis. Moreover, TAA also activates PLA2 which can increase lysolecithin and destray hepatocellular membrane. CCl4 is biotransformed into active trichloromethyl free radical by intrahepatocellular cytochrome P450, which may lead to lipid peroxide and injury of cell membrane. In brief, various pathogenic factors may all induce the liver damage by their own mechanism respectively.
When PLI is present, the structural and functional injuries of KCs occur at the same time with injuries of hepatic parenchymal cells. Our research indicated that mitochondria swelled, decreased and phagocytosis of KCs was markedly weakened during acute hepatic failure resulted from galactosamine or TAA. Other researchers observed KCs injury under electronmicroscopy at the first hour and almost all cells were necrotic at about the second hour after exposure to a lethal dose of ranine virus. The damage of hepatic parenchymal cells emerged at the fourteenth hour under the same circumstance. The function of mononuclear phagocyte system was inhibited in mice vaccinated with sub-lethal dose of ranine virus within 24 h in other reports[10].
Because of the serious destruction of tissues in severe hepatitis, KCs are overburdened in that they not only must phagocytize virus and immune complexes, but also remove cellular debris and other foreign bodies. Thus, the function of phagocytosis and elimination of endotoxin by KCs were impaired badly.
In physiological condition, the powerful phagocytosis of KCs require the help of nonspecific opsonin (such as fibronectin, Fn). In acute hepatic failure, the level of plasma Fn showed a significantly reduction due to the decline or loss of function that Fn is synthesized and secreted by hepatocytes. Experimental results revealed that there was a positive correlation between the decrease of Fn and phagocytosis of KCs[1]. In conclusion, IETM is further aggravated by the impaired phagocytosis of KCs after “Primary liver injury”
The SLI refers to the liver damage mediated by a battery of chemical mediators that were synthesized and released from KCs activated through IETM induced by PLI.
The SLI has been confirmed in a large number of animal experiments and clinical studies. First, In rats colectomized before administration of TAA, the level of plasmic endotoxin had no statistical difference from that in control group, the content of TNF-α was markedly lower and plasmic ALT activity was only one fifth of that in TAA group. Our findings indicated that severity of PLI induced by TAA was only one fifth of that by SLI[12].Second, it has been demonstrated in our extensive experiments that either inhibiting the function of KCs(GdCl3 and SiO2) or lightening IETM can attenuate the release of chemical mediators from KC and subsequent hepatic injury[10]. Third, it has been reported from plenty of domestic and foreign clinical data that there are a battery of chemical mediators, which were released by activated KC in plasma of patients with various hepatitis(derived from virus, alcohol etc.) and hepatic diseases (such as posthepatitic cirrhosis) in variable degrees, correspondingly brought about sorts of clinical signs and symptoms[13]. The “second liver injury” makes a great impact on the development and prognosis of hepatitis.
The plasma endotoxin level in patients with severe hepatitis was approximately five to eighttimes that of the normal person. The higher the level of endotoxin was, the severer the hepatitis would be. These data suggested that IETM had a great impact on the progress of hepatitis. KCs activated by LPS released a great deal of proinflammatory cytokines(TNF-α, IL-1, IL-6, etc.), lipid inflammatory mediators (LTs,PAF,TXA2,etc.), NO, endothelin and oxygen-derived free radicals, which further amplified the inflammatory reaction. TNF-α, the most crucial proinflammatory cytokine of all, could mobilize neutrophilic leukocytes chemofactically into liver and initiated a series of reactions, i.e. respiratory burst, release of oxygen-derived free radical, lipid peroxide and hepatocellular necrosis. It also could induce considerable intercellular adhesion molecule-1(ICAM-1) expression on sinusoidal endothelia and hepatocytes and promote CTLs to attack hepatocytes and led to massive hepatocellular necrosis. It was reported that there was a significantly positive correlation between TNF-α level and ICAM-1 mRNA content. Whereas ICAM-1 level also showed a significantly accordant positive correlation with serum ALT activity, the degree of hepatic necrosis, the degree of neutrophilic leucocytes infiltration in liver tissues. 67 percent of the necrotic area will be decreased if pretreated with anti-ICAM-1 antibody. These findings demonstrated that ICAM-1 played a vital role during the transformation of severe hepatitis into acute liver failure[6].
Liu et al[14] have observed that endotoxin-induced liver injury predominantly accounted for severe microcirculatory disturbance of liver. The response of hepatic microvasculum to LPS was directly related to the quantity and activated degree of KCs. The stronger the activated degree of KC was, the severer the hepatic microcirculatory disorder was. The histological observation showed that there was a large number of filiform ball microthrombi within hepatic sinusoids and micrangiums. The hemagglutination and the adhesion of activated leukocytes to vascular endothelium in sinusoids were observed too. The area of hepatic necrosis reached beyond 50 percent. The activity of plasmic ALT was significantly elevated. Obviously, the microcirculatory disturbance induced by LPS is predominantly responsible for hepatic persistent necrosis and the further development of acute liver failure. Ren et al[15] used intoxication by TAA to duplicate rat
model of severe hepatitis. The results indicated that in the TAA group, the level of plasmic endotoxin and the serum ALT activity were all significantly higher than those in the control group, the arterial ketone body ratio of acetoacetate to β-hydroxybutyrate(AKBR) decreased below 0.4, the ATP content of hepatocellular mitochondria was notably lower. While in the TAA plus colectomy group, there was no endotoxemia, the ATP content of hepatocellular mitochondria was sharply higher than that in the TAA group, but the serum ALT activity significantly lower than that in the TAA group. Thus, we might conclude that IETM played a key role in the occurrence of acute liver failure, the metabolic imbalance and dysfunction of liver might be caused by IETM through damaging hepatic energy metabolism.
We further investigated the role that functional state of KCs played in the occurrence of acute liver failure. The findings suggested that though a large single dose of CCL4 could induce severe injury of hepatic parenchymal cells, no liver failure occurred in rats, because the structure and function of KCs were not affected. At the twenty fourth hour after intragastric infusion of rats, the granular turbid fluid of silica (18 μg/100 g B.W.)was injected into caudal vein the level of plasma endotoxin was remarkably increasd due to inbibition of KCs. 0At the twenty seventh hour urine volume, excretion of urea nitrogen and creatinine dcereused markedly, with marked inerease of blood urea nitrogen and creatinine indicating renal failure. Obvious lethargic phenomenon in rats with electroencepholograph (EEG) showing specific triphasic wave of the early stage of hepatic coma was observed at the 36th hour. EEG presented large and slow alternative wave after 40th hour. These findings suggested hepatic coma had occurred in rats. All these results demonstrated that although there was not significant change in parenchymal liver cells, rats experienced hepatic failure quickly, presenting as renal failure and coma in comparision with rats intragastrically infused with CCl4 but without treatment with SiO2[16]. Similar results could be observesd in the model of fulminant hepatic failure induced by TAA. In rats intragastrically infused with TAA but preceded by injection of SiO2 in caudal vein, hepatic failure occurred rapidly. There was no significant difference in the injury extent of hepatic parenchymal cells as compared with rats without pretreatment with SiO2. These findings demonstrated that IETM induced by KCs dysfunction played a determinative role in promoting hepatic insufficiency to develop into hepatic failure[17].
In the presence of persistent and mild IETM, repeated and sustained hepatocellular injury and concomitant inflammatory cell infiltration frequently develops and transforming growth factor β (TGF-β) controls the repairment of liver tissues via restricting the regeneration of hepatocytes and increasing extracellular matrix (ECM). Long-term injection of CCl4 in small dose or oral intake of TAA in low concentration can cause chronic mild IETM with continous expression of TGF-β in the experiment rats which successively result in hepatic fibrosis, micronodular cirrhosis then hepatocarcinogenesis in part and hepatic failure often resulted from some inducing factors within 2-6 months.
Jia et al[18] had established rat model of hepatic fibrosis induced by compound factor(CCL4 mainly). Liver tissues were characterized by steatosis and necrosis attended by inflammatory infiltration within 1-2 weeks. Then hepatic fibrotic proliferation developed within 3-4 weeks. Hepatic fibrosis continuosly became aggravated, pseudulobuli were formed and hepatocirrhosis eventually developed within 5-8weeks. In the meanwhile, IETM became increasingly serious and the level of plasma endotoxin rose gradually, which was positively correlated with the amount of hepatic collagen. It was indicated through immunohisto chemical location that TNF-α was involved in the liver fibrosis mediated by endotoxin. By ultrastructural observation, the phenotype transformation of fat-storing cells suggested that functional state of fat-storing cells was closely related to endotoxin in the development of hepatic fibrosis.Cirrhosis which developed in rats drinking 0.03% TAA for four months was accompanied by IETM with higher plasma levels of TNF-α, endothelin, NO, MDA, and hepatic collagen contents simultaneously in experiment performed by Zhao et al[19]. Under electronmicroscopy, fat-storing cell nucleus showed the shape of star, cytoplasm projected outwardly, fat drop in cytoplasma diminished, its morphology was gradually transformed into that of myofibroblast. Collagen fibres apparently were increased around cell body. It was demonstrated by animal experiments that endotoxin could aggravate proliferation of hepatic fibers that developed into cirrhosis.
In the experiment by Yang et al[20,21]. 41.7 percent of rats drinking 0.03% TAA for 6 months developed hepatocarcinoma on the basis of cirrhosis with elevation of endotoxin. Endotoxin could increase both overexpressions of bcl-2, P53 protein and point mutation of N-ras, P53 genes; increase the generation of free radicals and reduce antioxidase activity with aggravation of injury of DNA. These results provided evidences that endotoxin could precipitate cancerous change induced by TAA.
In above experiments, drugs against intestinal endotoximia (such as herbal mixture “shuang Li Gan”)could alleviate cirrohsis, and bring about decreased incidence of hepatocarcinoma. It was demonstrated again that prevention and treatment of IETM could stop the prooress of hepatitis into chronicity.
Li et al have found that rats with cirrhosis became wilted somnolent and comatous and showed typical EEG changes at the fourth hour after a small dose of endotoxin injected once intraperitoneally. These manifestations suggested that hepatic encephalopathy (HE) had occurred in rats with cirrhosis. At this time blood ammonia level was elevated more than twice and plasma glucagons elevated more than ten times of that in normal rats. Glutamine contents of plasma and cerebrum were obviously higher than that in the cirrhotic control group. There were significant positive correlations between the levels of plasma endotoxin and plasma glucagon or ammonia, between the level of plasma ammonia and that of glutamine in cerebral homogenate. Apart from these, the contents of 5-hydroxytryptamin(5-HT),free tryptophan and 5-hydroxyindole acetic acid were all increased, whereas the contents of noradrenaline and dopamine were reduced in cerebral cortex.
It was exhibited from ultrastructural analysis that cellular swell and the diminished number of organellesin astrocytes of cerebral cortex markedly appeared in rats with HE induced by endotoxin. Immunohistochemical staining of TNF-α indicated that there was positive staining on endothelial cells of intracephalic micrangium and some astrocytes of cerebral cortex.
Based on the above results, HE in rats with cirrohsis induced by endotoxin might be associated with sharp rise of plasma glucagons and subsequent rise of blood ammonia. Owing to the high level of blood ammonia, a great deal of ammonia accumulated in cerebra and were transformed into glutamine under the action of glutamine synthetase of astrocytes. A great amount of glutamine was excreted from cerebra via blood-brain barriers (BBB) and resulted in a great amount of neutral amino acids transported into cerebra through the mechanism of carriers exchange. Great quantities of aromatic amino acids might inhibit tyrosine hydroxylase, and keep aromatic aminoacids (phenylalanine, tyrosine) from synthesizing true neurotransmitter-noradrenaline and dopamine, but transformed into false neurotransmitter. Besides, trytophan entering into the cerebra might form 5-HT under the action of corresponding enzyme. Thus, decreased true neurotransmitter with a coexisting increased inhibitory and(or) false neurotransmitter led to the functional inhibition of central nervous system followed by HE[22].
In summary, if PLI caused by various pathogenic factors through their independent specific mechanism is regarded as “the first hit” on liver, then SLI mediated by different chemical mediators from KCs activated by IETM in the course of hepatitis is “the second hit” on liver. Thus fusing and overlapping of the primary and secondary liver injuries determine and influence the complexity of the illness and outcome of the patient with hepatitis.
For this reason, the viewpoint of “secondary liver injury” induced by “the second hit” on liver inflicted by IETM suggests that medical professionals should attach great importance to both “primary liver injury”, and “secondary liver injury” caused by IETM. That is, try to adjust the function of KCs and eliminate endotoxemia of the patient. Thus, it is beneficial in the short term to the cure of hepatitis and prevention from developing to severe hepatitis. it facilitates blocking the risk of development from hepatitis to cirrhosis and hepatocarcinoma in the long term. So the defence line against liver failure should be constructed through clearing IETM.
Edited by Xu JY
| 1. | Zhao LF, Han DW. Clinical significance of endotoxemia in liver diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:391-393. |
| 2. | Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Hoek JB. Endotoxin and alcoholic liver disease: tolerance and susceptibility. Hepatology. 1999;29:1602-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Barton CC, Barton EX, Ganey PE, Kunkel SL, Roth RA. Bacterial lipopolysaccharide enhances aflatoxin B1 hepatotoxicity in rats by a mechanism that depends on tumor necrosis factor alpha. Hepatology. 2001;33:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Liang X, Qiao Z, Yin L. [Lipopolysaccharide-induced apoptosis of rat hepatocyte in vitro]. Zhonghua Ganzangbing Zazhi. 1999;7:72-73. [PubMed] |
| 6. | Ahmad N, Gardner CR, Yurkow EJ, Laskin DL. Inhibition of macrophages with gadolinium chloride alters intercellular adhesion molecule-1 expression in the liver during acute endotoxemia in rats. Hepatology. 1999;29:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Wall C, Raleigh JA, Frankenberg MV. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13 Suppl:S39-S50. [PubMed] |
| 8. | Stachlewitz RF, Seabra V, Bradford B, Bradham CA, Rusyn I, Germolec D, Thurman RG. Glycine and uridine prevent D-galactosamine hepatotoxicity in the rat: role of Kupffer cells. Hepatology. 1999;29:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Winwood PJ, Arthur MJ. Kupffer cells: their activation and role in animal models of liver injury and human liver disease. Semin Liver Dis. 1993;13:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Han DW. Studies on pathogenesis of hepatic failure: hypothesis of intestinal endotoxemia. Zhughua Ganzangbing Zazhi. 1995;3:134-137. |
| 11. | Han DW, Zhao LF. Effects of intestinal endotoxemia on chronic-ity of hepatitis. Jichu Yixue Yu Linchuang. 1999;19:482-487. |
| 12. | Liu L, Han D, Ren D. Effects of intestinal endotoxemia on pathogenesis of liver injury induced by thioacetamide. Zhonghua Ganzangbing Zazhi. 2000;8:174-175. [PubMed] |
| 13. | Han DW. Clinical basis for "secondary injury" induced by intestinal endotoxemia. Shijie Huaren Xiaohua Zazhi. 1999;7:1055-1058. |
| 14. | Liu LX, Han DW, Ma XH. Role of hepatic microcirculatory dis-turbance induced by intestinal endotoxemia in liver injury. Zhoughua Zhuanranbing Zazhi. 2001;19:94-96. |
| 15. | Ren DB, Han DW, Zhao YC. Effect of intestinal endotoxemia on hepatic energy metabolism in acute liver failure. Zhongguo Binglishengli Zazhi. 2001;17:890-892. |
| 16. | Han DW, Fu ST, Ma XH, Zhao YC, Yin L. The Role of Kuffer cells in acute liver failure. Zhonghua Ganzangbing Zazhi. 1994;2:71-74. |
| 17. | Jian SY, Han DW, Ma XH, Zhao YC, Yin L. Studies on actions of functional status of kupffer cells on experimental acute hepatic failure in rat. Zhughua Ganzangbing Zazhi. 1995;3:80-82. |
| 18. | Jia JB, Han DW, Xu RL, Chen XM, Zhao YC, Ma XH, Yan JP. Role of gut-derived endotoxemia in the pathogenesis of experi-mental hepatie fibrosis. Zhongguo Biualishengli Zazhi. 1998;14:396-399. |
| 19. | Zhao L, Li H, Han D. [Effects of intestinal endotoxemia on the development of cirrhosis in rats]. Zhonghua Ganzangbing Zazhi. 2001;9 Suppl:21-23. [PubMed] |
| 20. | Yang JM, Han DW, Liang QC, Zhao JL, Hao SY, Ma XH, Zhao YC. Effects of endotoxin on expression of ras, P53 and bcl-2 oncoprotein in hepatocarcinogenesis induced by thioacetamide in rats. Chin Natl new Gastroenterol. 1997;3:213-217. |
| 21. | Yang JM, Han DW, Xie CM, Liang QC, Zhao YC, Ma XH. Endotoxins enhance hepatocarcinogenesis induced by oral intake of thioacetamide in rats. World J Gastroenterol. 1998;4:128-132. [PubMed] |
| 22. | Li XQ, Han DW, Xu RL, Zhao YC, Ma XH. Hepatic encephal-opathy in cirrhotic rats induced by endotoxin injection. Zhongguo Binglishengli Zazhi. 1999;15:151-153. |