Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.1059
Revised: April 26, 2002
Accepted: May 11, 2002
Published online: December 15, 2002
AIM: To investigate the expression of bax, bcl-2 and bcl-xL mRNA in the tissues of normal liver and hepatocellular carcinoma (HCC), and analyze the relationship between the expression of bax, bcl-2 and bcl-xL mRNA and clinical parameters of HCC patients.
METHODS: The expression of bax, bcl-2 and bcl-xL mRNA of normal liver and HCC was measured by Northern blot. Statistical analyses were made by t test and correlation analysis.
RESULTS: A very low mRNA level was indicated at bax, bcl-2 and bcl-xL in the HCC tissues in contrast to the tissues of normal liver by Northern blot analysis. The analyses of mRNA level revealed that HCC tissues exhibited a mean 7.6-fold decrease in bax, 4.2-fold in bcl-2 and 3.5-fold in bcl-xL in comparison with normal control tissues, respectively. Positive correlation was found between bax and bcl-xL (r = 0.7061,P < 0.01). There was no significance between the mRNA expression of these three genes and age, gender, tumor differentiation and tumor stage of HCC patients .
CONCLUSION: The results are consistent with the fact that apoptosis rarely occurs in normal livers but increases in HCC, indicating that bcl-2 and bcl-xL may play a very important role in regulating the apoptosis of normal liver and HCC.
- Citation: Guo XZ, Shao XD, Liu MP, Xu JH, Ren LN, Zhao JJ, Li HY, Wang D. Effect of bax, bcl-2 and bcl-xL on regulating apoptosis in tissues of normal liver and hepatocellular carcinoma. World J Gastroenterol 2002; 8(6): 1059-1062
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/1059.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.1059
Apoptosis is a highly regulated form of programmed cell death defined by distinct morphological and biochemical features. Programmed cell death is involved in a type of cell death, in which the cell actively uses a genetically controlled program to cause its own demise during the tissue remodeling of embryogenesis[1]. Apoptosis is a key mechanism causing cell death and organ diseases, failure of apoptosis is now understood to contribute to the development of human malignancies[2-5].
The bcl-2 family is the best characterized group of apoptosis-mediating factors, which include bcl-2, mcl-1, bcl-x, bax, bak, and several others. Although its members share close structural homologies, their biologic functions differentiate into apoptosis-promoting (bax, bak, bcl-xS) or apoptosis-inhibiting (bcl-2, mcl-1, bcl-xL) properties[1,6]. The bcl-2 related genes regulate cell death and are considered to correlate with the pathogenesis and progression of cancers[7-14].Since the relationship between bcl-2 family and HCC is still unclear, we investigated the expression of the three genes in HCC and normal controls and evaluated the mediating action in HCC and the relationship between the genes and clinical parameters as well.
Ten cases of (4 women, 6 men) normal liver tissues were obtained. The median age in the control group was 57 years, with a range of 39-75 years. HCC tissues were obtained from 21 patients (9 women, 12 men) undergoing surgery for HCC. The median age of the HCC patients was 64 years (a range of 33-76 years). According to the TNM classification of the International Union against Cancer, there were 6 patients with stage II,13 with stage III, and 2 with stage IVdisease. Tissues destined for RNA extraction were frozen in the operating room in liquid nitrogen immediately on surgical removal and maintained at -80 °C until use.
Total RNA was extracted by the guanidinium isothiocyanate method, fractionated on 1.2% agarose. 1.8 mol·L formaldehyde gels, and stained with ethidium bromide for verification of RNA integrity and loading equivalence[15]. The RNA was electrotransferred to Nylon membranes (GeneScreen; DuPont, Boston, Massachusetts, USA) and cross linked by UV irradiation. The filters were then prehybridized, hybridized, and washed under conditions appropriate for digoxigenin labelled antisense riboprobes (bcl-2 and bax) or the 32P labelled antisense riboprobe (bcl-xL) and cDNA probe (7S) as previously described[15,16].
In the case of the digoxigenin-labelled bcl-2 and bax cRNA probes, the filters were prehybridized and hybridized overnight at 68 °C (bcl-2) or 65 °C (bax) in a buffer containing 50% formamide, 2 × SSC (1 × SSC is 0.15M NaCl/0.015 M sodium citrate buffer) (bcl-2) or 5 × SSC (bax), 2% blocking reagent (Boehringer Mannheim, Mannheim, Germany), 0.1% N-lauroylsarcosine, and 0.02% sodium dodecyl sulphate (SDS). The filters were then washed in 2 × SSC/0.1% SDS at room temperature, followed by three 15 min washing at 68 °C (bcl-2) or 65 °C (bax) in 0.065 × SSC/0.065% SDS/35% formamide (bcl-2) or 0.1 × SSC /0.1% SDS (bax). The filters were then incubated in a blocking buffer (1% blocking reagent in 100 mmol·L maleic acid/150 mmol·L NaCl, pH 7.5) for 30 min, and in blocking buffer containing anti-digoxigenin alkaline phosphatase antibody (1:20000; Boehringer Mannheim ) for 30 min, washed three times with maleic acid buffer for 15 minutes, and incubated with 25 mmol/L CDP-Star (Boehringer Mannheim). The membranes were then exposed to X ray films.
For the 32P labelled antisense riboprobe (bcl-xL), the blots were prehybridized for 6 hours in 50% formamide,0.5% sdiumm dodecyl sulfate (SDS), 5 × SSC (sodium chloride/ sodium citrate buffer), 5 × Denhardt’s solution (1 × Dennhard’s solution = 0.02% ficoll, 0.02% polyvinylpyrrolidone, and 0.02% bovine serum albumin), 250 mg/L salmon sperm DNA, and 50 mmol/L sodium phosphate buffer (pH 6.5). The blots were then hybridized for 18 hours at 65 °C in the presence of 1 × 106 cpm/ml labeled antisense riboprobe, washed twice at 65 °C in a solution containing 1 × SSPE (150 mmol/1 NaCl, 10 mmol/1 NaH2PO4, and 1 mmol/1 EDTA) and 0.5% SDS, and twice at 65 °C in a solution containing 0.1 × SSPE and 0.5% SDS.
In the case of the 7S cDNA probe, blots were prehybridized for eight hours at 42 °C in a buffer which contained 50% formamide, 1% SDS, 0.75 mol/L NaCl, 5 mmol/L EDTA, 5 × Denhardt’s solution, 100 mg/L salmon sperm DNA, 10% dextran sulfate, and 50 mmol/1 phosphate buffer (pH 7.4). The hybridization was carried out at 42 °C for 18 hours by adding the 32P labelled cDNA probe (1 × 105 cmp/mL). The blots were rinsed twice in 2 × SSC at room temperature and washed three times at 55 °C in 0.2 × SSC/2% SDS under conditions appropriate for cDNA probes. Blots were then exposed at -80 °C to Fuji X ray films with intensifying screens (DuPont).
For statistical analysis of the Northern blot results, the intensity of the radiographic bands was quantified by laser densitometry (Bio-Rad 620; Richmond, Califirnia, USA). The ratio between the bax, bcl-2 or bcl-xL and the corresponding 7s signal was calculated in each sample.
The data were expressed as median and range. Statistical analyses were carried out using the SPLM software (Statistical Department of Fourth Military Medical University). For statistical analysis, the t test and correlation analysis were used. Significance was defined as P < 0.05.
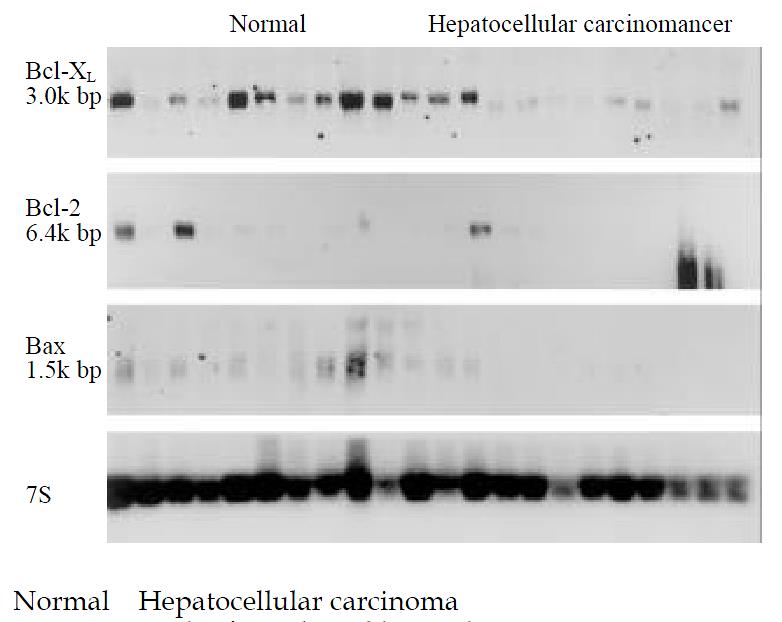
Northern blot analysis was carried out to determine bax, bcl-2 and bcl-xL mRNA expression in the normal and the cancerous liver. In contrast, hybridization signals of mRNA of three genes were higher in normal liver tissues as compared with the signals in cancerous samples (Figure 1). A low mRNA level of bax, bcl-2 and bcl-xL mRNA was almost present in all HCC tissue samples. In some HCC tissues the expression levels for all three genes were very faint and were only visible on the original autoradiographs. None of the normal or cancer samples showed any aberrant bax, bcl-2 or bcl-xL mRNA transcripts, and bax and bcl-2 mRNA were reduced in 100% of cancer samples, and bcl-xL mRNA was reduced in 95% of cancer samples in contrast with the normal controls. Densitometric analysis of the Northern blots indicated that the bax and bcl-2 mRNA levels in all cancer samples were 7.6- and 5.4-fold (P = 0.0002; P = 0.00887) higher than those in the matched control samples; bcl-xL mRNA levels in all cancer samples were 3.5-fold lower (P = 0.0002) than those in the normal samples (Table 1). The expression of bax and bcl-xL mRNA was positively correlated in HCC (r = 0.7061, P < 0.01), but there was no correlation between bax and bcl-2 or bcl-2 and bcl-xL (r = 0.1637, r = 0.4830).
| Hepatocellular carcinoma (21 cases) | normal liver (10 cases) | t value | P value | |
| Bax | 0.929 ± 1.233 | 7.060 ± 6.574 | 4.197 | 0.0002 |
| Bcl-2 | 1.414 ± 1.331 | 5.930 ± 7.227 | 2.815 | 0.0087 |
| Bcl-xL | 2.433 ± 2.218 | 8.500 ± 5.743 | 4.277 | 0.0002 |
To determine whether the presence of bax, bcl-2 or bcl-xL mRNA in the HCC tissues is of clinical significance, the Northern blot data were statistically analysed in patient data (sex, age) and clinical data (tumor stage, tumor differentiation). No significance was found between the expression of bax,bcl-2 or bcl-xL and these parameters (Table 2).
| n | bax | bcl-2 | bcl-xL | ||
| Age | ≤ 65 | 10 | 0.980 ± 1.530 | 1.960 ± 1.678 | 3.020 ± 2.549 |
| > 65 | 11 | 0.882 ± 0.962 | 0.918 ± 0.663 | 1.900 ± 1.825 | |
| gender | male | 13 | 1.100 ± 1.471 | 1.654 ± 1.532 | 2.600 ± 2.645 |
| female | 8 | 0.650 ± 0.699 | 1.025 ± 0.871 | 2.163 ± 1.386 | |
| grading | well and moderate | 12 | 0.975 ± 1.384 | 1.592 ± 1.540 | 2.775 ± 2.453 |
| poor and undifferentiated | 9 | 0.867 ± 1.076 | 1.17 ± 1.028 | 1.978 ± 1.901 | |
| tumor stage | I II | 6 | 0.583 ± 0.454 | 0.700 ± 0.616 | 2.000 ± 1.616 |
| III IV | 15 | 1.067 ± 1.423 | 1.700 ± 1.445 | 2.607 ± 2.445 |
Apoptosis is a central regulator of tissue homeostasis. It contributes to the elimination of damaged cells in normal tissues and balances the appropriate cell number under the circumstances of physiologic cell proliferation and tissue repair. In the past few years, scientific interest has focused on the process of apoptosis. Like cell replication, apoptosis is controlled by the network of positive and negative growth signals. Based on the currently prevailing views, it is assumed that malignant cells should be incapable of apoptosis and/or not responsive to death signals, thereby allowing unrestrained growth of cancer. Recently, more and more studies indicated that apoptosis is of importance in the growth and development of many tumors, but the pattern of apoptosis varies in different tumors[11-19]. In contrast with normal tissues, there is often reduction of apoptosis in most cancerous tissues[20-23], but hepatic cancer is different. HCC is one of the most common and aggressive tumors in the world today, and little is known about the cellular pathogenesis[24-34]. The apoptosis is rare in normal liver tissues (there is only 2-4 apoptotic cells per 10000 hepatic or biliary cells[35]), while the HCC tissues have higher rates of apoptosis[37].These findings indicate that the apoptosis-related genes are expressed in various frequencies in different cancers, and a general pattern of activation or inactivation of these genes in malignant tumors cannot be defined. Therefore, the function of apoptotic genes in different human cancers must be evaluated individually.
In this study, we analysed the concomitant expression of bax, bcl-2 and bcl-xL in the HCC tissues and normal liver. These genes belong to the same family of apoptotic genes. Although the structures are similar, they exert opposite effects on apoptosis. bcl-2 and bcl-xL inhibit apoptosis and contribute to cell survival and the resistance of cells against damaging influences. In contrast, bax, which is considered to be a central regulator of apoptosis, is a promoter of programmed cell death[37]. The relationship between bcl-2 related genes and HCC is still unclear.
By the analysis of 21 HCC patients , reduced bax and bcl-2 signals were present in all samples and reduced bcl-xL signals were present in 95% of the cancer samples. The low expression of bcl-2 and bcl-xL is consistent with enhanced apoptosis in the HCC, but the low expression of bax does not account for this phenomenon. The findings revealed that anti-apoptotic genes, but not apoptosis-promoting genes, might play a more important role in regulating the apoptosis of normal liver and HCC. There was no correlation between bax, bcl-2 and bcl-xL and age, gender, differentiation or stage of tumor in HCC patients. We can not give a reasonable explanation for the finding that the expression of bax and bcl-xL was positively correlated in HCC. We concluded that anti-apoptotic genes do not influence the differentiation and development of HCC, but these genes can increase the rate of apoptosis in HCC by reducing their expression or changing the ratio with other genes. The influencing factors of apoptosis increased in HCC are not known, which may include 1) inherent metabolic process of tumor tissues;2) hypoxia of tumor tissues;3) some cytokines such as tumor necrosis factor;4) attack of CTL, etc.
The low expression of anti-apoptotic genes, which increase apoptosis in HCC, may have negative impact on growth of tumors as a homeostasis mechanism that inhibits cell group with extensive growth, and this effect can delay the development of tumors. But it may also be a selective pressure, which removes the aging tumor cells or some tumor cells with phenotype similar to normal cells and selects more aggressive and prosperous clone of tumor cells, accelerating the development of tumors. Further studies on the regulating action of apoptotic genes will deepen the understanding about the growth and development of HCC and offer valuable information to genetic therapy of HCC, thus enhancing the sensibility to radiotherapy and chemotherapy.
Edited by Ma JY
| 1. | Que FG, Gores GJ. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Friess H, Lu Z, Graber HU, Zimmermann A, Adler G, Korc M, Schmid RM, Büchler MW. bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut. 1998;43:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Deng LY, Zhang YH, Xu P, Yang SM, Yuan XB. Expression of IL 1betaconverting enzyme in 5-FU induced apoptosis in esophageal carcinoma cells. World J Gastroenterol. 1999;5:50-52. [PubMed] |
| 4. | Zhao AG, Yang JK, Zhao HL. Chinese Jianpi herbs induce apoptosis of human gastric cancer grafted onto nude mice. Shijie Huaren Xiaohua Zazhi. 2000;8:737-740. |
| 5. | Xin ZP, Xu CF, Huang YX, Wen QS, Xu CZ, Zhao YF. Experi-mental study on induction of apoptosis by cisplatin in human gastric carcinoma cell line SGC-7901. Huaren Xiaohua Zazhi. 1998;6:844-846. |
| 6. | Friess H, Lu Z, Andrén-Sandberg A, Berberat P, Zimmermann A, Adler G, Schmid R, Büchler MW. Moderate activation of the apoptosis inhibitor bcl-xL worsens the prognosis in pancreatic cancer. Ann Surg. 1998;228:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421-425. [PubMed] |
| 8. | Wu JY, Zhou XF, Jiang WX, Wang JL, Yang F, Cai XS, Zhang ZG. The impact of chemotherapeutic drugs on the expression of bcl-2,p53 and Ki-67 in gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:589. |
| 9. | Yuan RW, Ding Q, Jiang HY, Tan XF, Zou SQ, Xia HS. Apoptosis and the expression of bcl-2 and p53 in pancreatic cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:851-854. |
| 10. | Qiao Q, Wu JS, Zhang J, Ma QJ, Lai DN. The expression of bcl-2 and bax in human colon cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:936-938. |
| 11. | Chen XJ, Ai ZL, Liu ZS. Apoptosis-study on the mechanism of hepatic cell injury by mitomycin. Shijie Huaren Xiaohua Zazhi. 2000;8:746-750. |
| 12. | Yang JQ, Yang LE, Zhu HC. Mitomycin induces apoptosis of human hepatic cancer cell. Shijie Huaren Xiaohua Zazhi. 2001;9:268-272. |
| 13. | Guo WJ, Yu EX, Zheng SG, Shen ZZ, Luo JM, Wu GH, Xia SA. Study on Jianpi Liqi medicine inducing apoptosis in human he-patic cancer cell SMMC7721. Shijie Huaren Xiaohua Zazhi. 2000;8:52-55. |
| 14. | Chen HY, Liu WH, Qin SD. Induction of arsenic trioxide on apoptosis of hepatic cancer cell strain. Shijie Huaren Xiaohua Zazhi. 2000;8:532-535. |
| 15. | Liang WJ, Huang ZY, Ding YQ, Zhang WD. Lovo cell line apoptosis induced by cycloheximide combined with TNFα. Shijie Huaren Xiaohua Zazhi. 1999;7:326-328. |
| 16. | Lin JK, Chou CK. In vitro apoptosis in the human hepatoma cell line induced by transforming growth factor beta 1. Cancer Res. 1992;52:385-388. [PubMed] |
| 17. | Tu SP, Jiang SH, Tan JH, Jiang XH, Qiao MM, Zhang YP, Wu YL, Wu YX. Proliferation inhibition and apoptosis induction by ar-senic trioxide on gastric cancer cell SGC-7901. Shijie Huaren Xiaohua Zazhi. 1999;7:18-21. |
| 18. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4724] [Cited by in RCA: 4686] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 19. | Fukuda K, Kojiro M, Chiu JF. Demonstration of extensive chromatin cleavage in transplanted Morris hepatoma 7777 tissue: apoptosis or necrosis. Am J Pathol. 1993;142:935-946. [PubMed] |
| 20. | Xiao B, Xiao LC, Lai ZS, Zhang YL, Zhang ZS, Zhang WD. In vitro experimental study on Zhen’ailing inhibiting growth of gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:951-954. |
| 21. | Li LP, Zhang Z, Han SX. Retinoic acid inhibits proliferation of tumor cells and induces apoptosis. Shijie Huaren Xiaohua Zazhi. 2001;9:437-440. |
| 22. | Liu HF, Liu WW, Fang DC. Anti-Fas monoclonal antibody in-duces apoptosis of human gastric cancer cell line SGC-7901.. Shijie Huaren Xiaohua Zazhi. 1999;7:476-478. |
| 23. | Zhan J, Xie DR, Yao HR, Lin XG, Liang XW, Xiang YQ. Arsenic trioxide induces apoptosis of colon cancer line SW620. Shijie Huaren Xiaohua Zazhi. 2001;9:228-229. |
| 24. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. The expression of bax in gastric precancerous and cancerous tissues and its rela-tion with apoptosis. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. |
| 25. | Xu AG, Li SG, Liu JH, Gan AH. The function of apoptosis and protein expression of bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2000;6:27. |
| 26. | Lai DN, Wu JS, Wu YZ. The relationship between the expression of bcl-2 and differentiation of colon and rectum cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:91-92. |
| 27. | Soini Y, Virkajärvi N, Lehto VP, Pääkkö P. Hepatocellular carcinomas with a high proliferation index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br J Cancer. 1996;73:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Friess H, Yamanaka Y, Büchler M, Berger HG, Kobrin MS, Baldwin RL, Korc M. Enhanced expression of the type II transforming growth factor beta receptor in human pancreatic cancer cells without alteration of type III receptor expression. Cancer Res. 1993;53:2704-2707. [PubMed] |
| 29. | Guo X, Friess H, Graber HU, Kashiwagi M, Zimmermann A, Korc M, Büchler MW. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996;56:4876-4880. [PubMed] |
| 30. | Zang GQ, Yu H, Zhou XQ, Liao D, Xie Q, Wang B. TNF- in vitro induces apoptosis and necrosis of rat hepatic cell. Shijie Huaren Xiaohua Zazhi. 2000;8:303-306. |
| 31. | James SJ, Muskhelishvili L. Rates of apoptosis and proliferation vary with caloric intake and may influence incidence of spontaneous hepatoma in C57BL/6 x C3H F1 mice. Cancer Res. 1994;54:5508-5510. [PubMed] |
| 32. | Mills JJ, Chari RS, Boyer IJ, Gould MN, Jirtle RL. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995;55:979-983. [PubMed] |
| 33. | Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996;56:3391-3394. [PubMed] |
| 34. | Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology. 1995;21:1725-1741. [PubMed] [DOI] [Full Text] |
| 35. | Peng LM, Wang ZL. Foundation and clinic of apoptosis. 1st eds. Beijing: Renmin Weisheng Chubanshe. 2000;394. |
| 36. | Grasl-Kraupp B, Ruttkay-Nedecky B, Müllauer L, Taper H, Huber W, Bursch W, Schulte-Hermann R. Inherent increase of apoptosis in liver tumors: implications for carcinogenesis and tumor regression. Hepatology. 1997;25:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Walton MI, Whysong D, O'Connor PM, Hockenbery D, Korsmeyer SJ, Kohn KW. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993;53:1853-1861. [PubMed] |