INTRODUCTION
The induction of tumor cell differentiation is a new strategy of drug therapy of tumors. In recent years, many studies showed that malignant tumor cells could be induced to terminal differentiation by some inducers. Differentiation-inducing therapy has already been applied to leukemias, lymphomas, and other solid tumors[1]. On the other hand, the study on antitumor activities of marine bioactive substances has became an important field in exploiting marine bioactive substances and antitumor drugs[2-4]. In order to explore the method and means of regulating the proliferation of tumor cell and reversing its malignant phenotypes artificially, we focus on screening and identifying marine bioactive substances, especially those with low molecular weight which can adjust cell signal transduction and regulate cell proliferation and differentiation.
Horseshoe crab, a kind of marine animal, which has survived relatively unchanged for the past 350 million years, now is at the center of a resource tug of war. During the past two decades, many bioactive substances with special functions, including clotting factors, lectins, proteinase inhibitors, defensins, tachyplesin and tachystatins, have been found in the hemocytes and hemolymph plasma of horseshoe crab[5-9]. In our previous works, we reported that tachyplesin could alter the malignant morphological and ultrastructural characteristics and regulate the proliferation and differentiation of human gastric carcinoma cells[10,11]. In this paper, the antitumor activities of tachyplesin on hepatocellular carcinoma cell line SMMC-7721 were investigated to further clarify the antitumor mechanism of tachyplesin.
MATERIALS AND METHODS
Drugs and reagents
Tachyplesin was isolated from acid extracts of Chinese horseshoe crab (Tachypleus tridentatus) hemocytes as described by Nakamura et al[12]. RPMI-1640 medium were obtained from Gibco. Fetal calf serum was supplied by Si-Ji-Qing Biotechnology Co. (Hangzhou, China). Trypan blue were purchased from Sigma Co. γ-GT kit was purchased from Shanghai Lianyang Biotechenical Co. Mouse anti-human α-FP, PCNA, p21WAF1/CIP1, c-myc monoclonal antibodies were purchased from Santa Cruz. SP detection kit and DAB kit were purchased from Beijing Zhongshan Biotechnology Co.
Cell culture and treatment
SMMC-7721 cells, provided by the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, were maintained in RPMI-1640 medium supplemented with 20% heat-inactivated fetal calf serum, 100 units/mL penicillin, 100 mg/L streptomycin and 50 mg/L kanamycin at 37 °C, 5% CO2 in air atmosphere. SMMC-7721 cells were treated with culture medium containing tachyplesin after being seeded for 24 h.
Determination of cell growth curve and cell mitotic index
SMMC-7721 cells (5 × 104/mL) were seeded in small culture flasks or in little penicillin bottles with cover slips respectively. Tachyplesin treatment was performed after cells being subcultured for 24 h. From the first to seventh day, three flasks of untreated or tachyplesin-treated cells were harvested every day, and the viable cells were counted by the trypan blue dye exclusion test. Meanwhile, the cover slips with untreated or tachyplesin-treated cells were also taken out every day, fixed in Bouin-Hollande fixative, and stained with Hematoxylin-Eosin (HE) staining. The mitotic cells in 1000 cells on each cover slip were counted.
Sample preparation for the light microscopy
SMMC-7721 cells and the cells treated with 3.0 mg/L tachyplesin for 5 days were seeded in little penicillin bottles with cover slips, and grown in the normal culture medium or in the medium containing 3.0 mg/L tachyplesin for 48 h, respectively. The cells on cover slips were rinsed with D-Hank’s solution twice at 37 °C, fixed overnight in Bouin-Hollande fixative, stained with Hematoxylin-Eosin staining, and observed under light microscope.
Sample preparation for transmission electron microscopy
SMMC-7721 cells and the cells treated with 3.0 mg/L tachyplesin for 7 days were rinsed with D-Hank’s solution twice at 37 °C, shaved into centrifuge tubes with plastic scraper. Cells were centrifuged at 2000 rpm for 15 min, and the supernatants were removed. The precipitates were prefixed in 2.5% glutaraldehyde for 2 h and postfixed in 1% osmian tetroxide for 2 h, dehydrated in ethanol series, embedded in epoxy resin 618, stained with lead citrate and uranyl acetate, and observed under the JEM-100CXII transmission electron microscope.
Assays for the activities of γ-glutamyltransferase (γ-GT) and tyrosine aminotransferase (TAT)
SMMC-7721 cells and the cells treated with 3.0 mg/L tachyplesin for 7 days were harvested, counted, and sonicated in ice-cooled 0.1 mol/L PBS. The homogenates were centrifuged and the supernatants were subjected to assay of enzymatic activities. The γ-GT activity was determined with γ-GT reagent kit and the TAT activity was detected according to the method described by Marston et al[13]. The activities of γ-GT and TAT were valued as OD unit per 1 × 106 cells.
Immunocytochemistry analysis
SMMC-7721 cells and the cells treated with 3.0 mg/L tachyplesin for 5 days were seeded in little penicillin bottles with cover slips for 48 h respectively. The cells grown on cover slips were fixed with cold acetone for 10 min, rinsed twice in PBS for 15 min. They were then immersed in 3% hydrogen peroxide for 10 min, washed with distilled water and PBS for 15 min, blocked with 10% normal goat serum for 10 min at room temperature, and incubated with the monoclonal mouse anti-human α-FP, PCNA, p21WAF1/CIP1, c-myc antibodies at 4 °C overnight. After incubation with primary antibodies, cover slips were rinsed twice in PBS for 15 min, incubated with biotin-labeled secondary antibody at 37 °C for 10 min, rinsed twice in PBS for 15 min, and then incubated in streptavidin-peroxidase at 37 °C for 10 min. The antigen-antibody complex was visualized with diaminobenzidine (DAB) substrate. Negative controls were incubated in the absence of primary antibodies.
Statistical analysis
Student’s t test was used to compare the difference between control group and tachyplesin treatment group. The data of cell growth, mitotic index and enzymatic activities were presented as the mean with the corresponding standard deviation. P value of less than 0.05 is considered statistically significant.
RESULTS
Effects of tachyplesin on the proliferation of SMMC-7721 cells
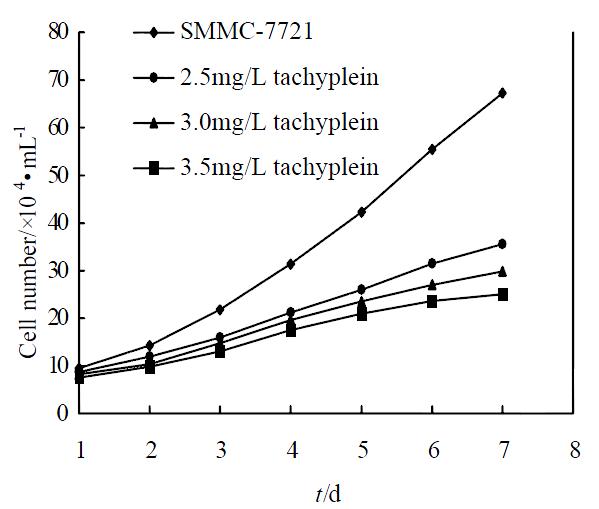
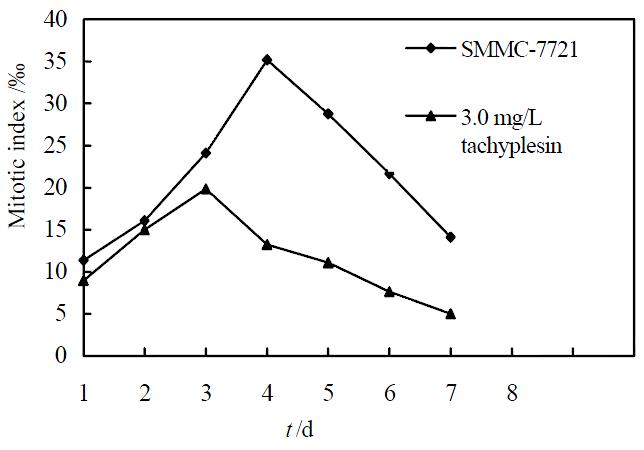
Under concentration of 2.5, 3.0, 3.5 mg/L, tachyplesin significantly inhibited the proliferation of SMMC-7721 cells. The growth inhibitory rates on SMMC-7721 cells on the seventh day were 47.09%, 55.57% and 62.77%, respectively (P < 0.05) (Figure 1). According to the principle of induced differentiation treatment, we chose 3.0 mg/L tachyplesin for induced treatment. Cell mitotic index determination showed that SMMC-7721 cells had vigorous proliferation capability, reached to the divided peak on the fourth day, and the maximum mitotic index amounted to 35.19‰± 2.17‰. However the mitotic index of the cells treated with 3.0 mg/L tachyplesin was only 19.82‰± 1.89‰ at the divided peak, declined by 43.68% (P < 0.05), and the divided peak was on the third day after tachyplesin treatment (Figure 2).
Figure 1 Effect of tachyplesin on the growth of SMMC-7721 cells.
Figure 2 Effect of tachyplesin on the mitotic index of SMMC-7721 cells.
Effects of tachyplesin on the morphology and ultrastructure of SMMC-7721 cells
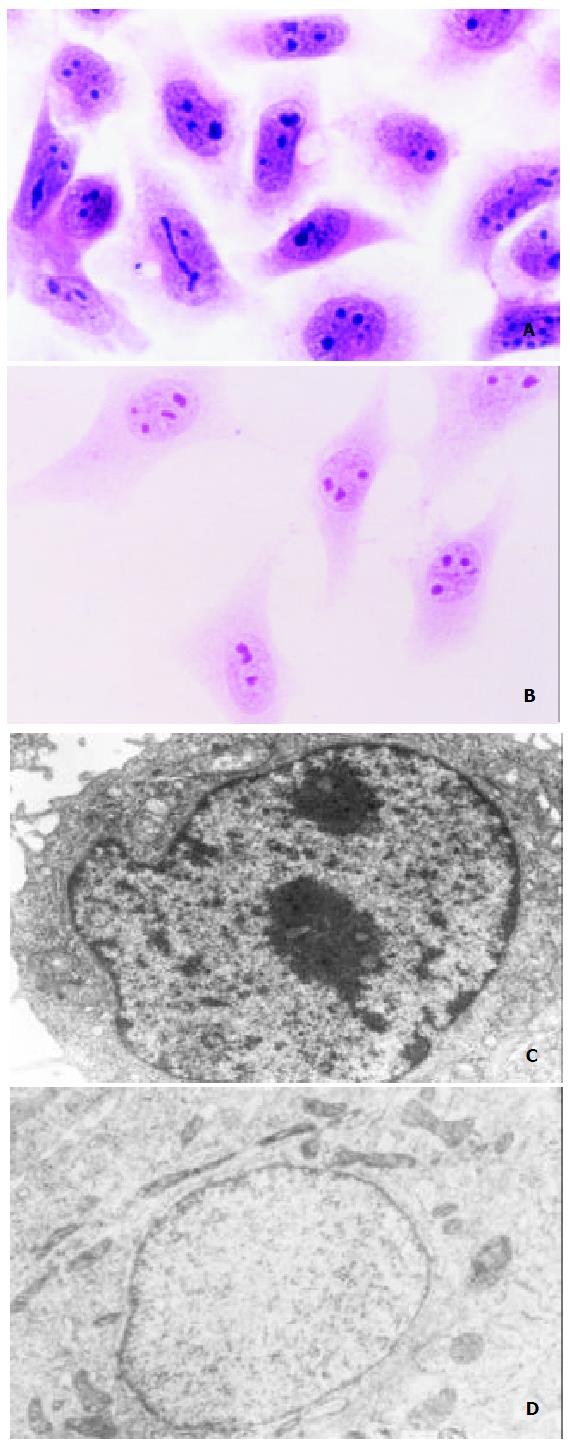
SMMC-7721 cells showed typical malignant morphological and ultrastructural characteristics in SMMC-7721 cells like other epithelial cancer cells. Under light microscope, the volume of SMMC-7721 cells was relatively small, nuclei were large and irregular with several nucleoli in it, and cytoplasm volume was small (Figure 3A). And it was revealed by transmission electron microscope that the necleo-cytoplasmic ratio of SMMC-7721 cells was relatively large, nuclei were irregular with many heterochromatins and few euchromatins and large nucleolus in it, while cell organelles were not well-developed in the cytoplasm (Figure 3C). However, after being treated with 3.0 mg/L tachyplesin, SMMC-7721 cells had undergone a significant morphological and ultrastructural changes and appeared as normal differentiated epithelial cells. The cells turned to be more spread and flat, the necleo-cytoplasmic ratio was reduced, the shape of nuclei became regular, heterchromatin in nucleus decreased while euchromatin increased, the volume of nucleolus was reduced, and cytoplasm was abundant with well-developed cell organelles (Figure 3B, D).
Figure 3 Effects of tachyplesin on the morphology and ultra-structure of SMMC-7721 cells.
A: SMMC-7721 cells ( × 536); B: SMMC-7721 cells treated with tachyplesin (× 536); C: The nucleo-cytoplasm ratio is large, the shape of nucleus is irregu-lar and cell organelles are not well-developed in the cytoplasm of SMMC-7721 cell (× 8800); D: The nucleo-cytoplasm ratio decreased, the shape of nucleus is regular and cell organelles are well-developed in the cytoplasm of tachyplesin-treated SMMC-7721 cell ( × 11000).
Effects of tachyplesin on the activities of γ-GT and TAT in SMMC-7721 cells
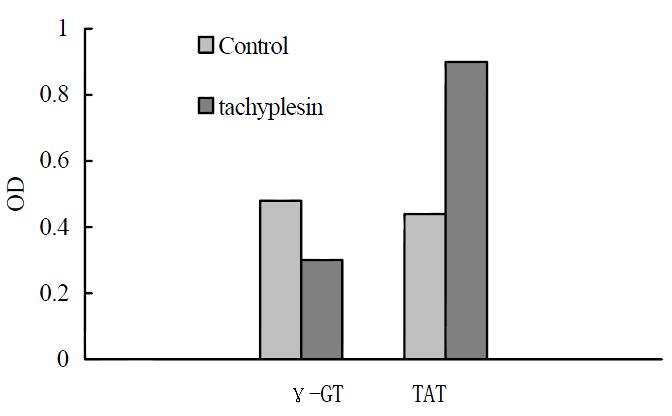
The human hepatocarcinoma SMMC-7721 cells had high γ-GT activity and low TAT activity. After treatment with 3.0 mg/L tachyplesin, the activity of γ-GT in SMMC-7721 cells were declined from 0.48 ± 0.04 Unit to 0.30 ± 0.02 Unit (P < 0.05) while TAT activity increased from 0.44 ± 0.04 Unit to 0.90 ± 0.04 Unit (P < 0.05) as shown in Figure 4.
Figure 4 Effects of tachyplesin on the activies of γ-GT and TAT in SMMC-7721 cells.
Effects of tachyplesin on α-FP, PCNA levels in SMMC-7721 cells
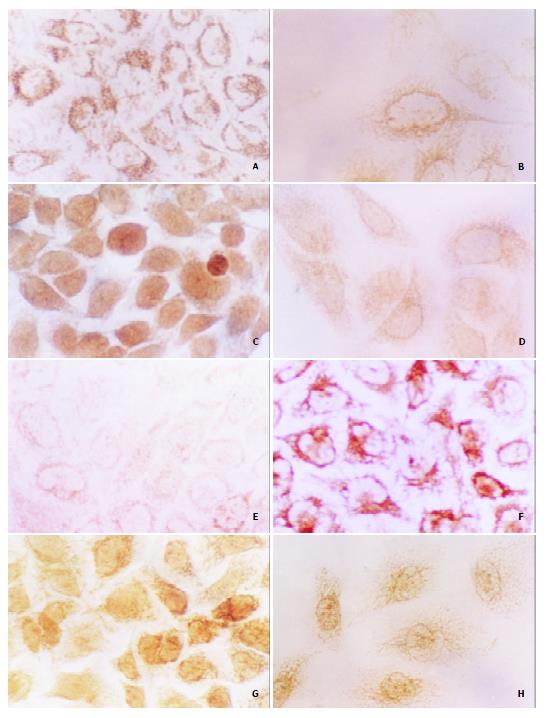
High level of α-FP was detected in the cytoplasm and nucleus of SMMC-7721 cells (Figure 5A). However, after being treated with 3.0 mg/L tachyplesin, the cells had a very low level of α-FP (Figure 5B). The PCNA level was also high and mainly distributed in the nucleus of SMMC-7721 cells, especially in the spherical cells, while the immunocytochemical signal was weak in the tachyplesin-treated cells (Figure 5C, D).
Figure 5 Effects of tachyplesin on the levels of α-FP, PCNA, p21WAF1/CIP1, c-myc proteins in SMMC-7721 cells ( × 536).
A: The high level of α-FP in SMMC-7721 cells detected by immunocytochemistry; B: The level of α-FP in the tachyplesin-treated SMMC-7721 cells is decreased; C: The high level of PCNA in SMMC-7721 cells; D: The level of PCNA in the tachyplesin-treated SMMC-7721 cells is decreased; E: The low level of p21WAF1/CIP1 in SMMC-7721 cells; F: The level of p21WAF1/CIP1 in the tachyplesin-treated SMMC-7721 cells is increased; G: The high level of c-myc in SMMC-7721 cells; H: The level of c-myc in the tachyplesin-treated SMMC-7721 cells is decreased.
Effects of tachyplesin on protein p21WAF1/CIP1, c-myc levels in SMMC-7721 cells
To investigate the effects of tachyplesin on the expression of differentiation-associated tumor suppressor gene and oncogene, the levels of p21WAF1/CIP1 and c-myc proteins were also examined. Immunocytochemistry showed that the level of p21WAF1/CIP1 protein was low in the nucleus and cytoplasm of the untreated cells while it was very high in the cells treated with tachyplesin (Figure 5E, F). High level of c-myc was observed in SMMC-7721 cells and exposure of the cells to tachyplesin resulted in an obvious decrease of c-myc protein (Figure 5G, H).
DISCUSSION
It is important to examine the changes of the main malignant characteristics of cancer cells in order to evaluate the malignant phenotype reversion. Continual division and constant proliferation are important characteristics of tumor cells. The proliferating activity of cells is inversely correlated with the degree of differentiation. Therefore, inhibiting the proliferation of tumor cells is a significant index in induction of differentiation[1,14]. In the present study, the results of the cell growth curve and mitotic index assay indicated that tachyplesin could inhibit the multiplicative activity of hepatocarcinoma cells effectively, with similar effects of growth inhibition as retinoic acid, HMBA and dimethyl sulphoxide on human hepatocarcinoma cells HepG2 and SMMC-7721[15-20]. It suggests that tachyplesin could suppress tumor cell growth as other differentiation inducers.
Malignant morphology and ultrastructure are other important characteristics of tumor cells. Previous studies had revealed that lots of differentiation inducers could reverse the morphology and ultrastructure of tumor cells. Therefore, evaluating the changes of these two characteristics in tumor cells is important in determining the effects of exotic substances, especially differentiation inducers on tumor cells. Our results showed that tachyplesin could reverse the malignant morphological and ultrastructural characteristics of SMMC-7721 cells as it did on gastric carcinoma cells or other inducers on hepatocarcinoma cells and other tumor cells[10,18,19].
α-FP, an oncofetal antigen, is as an important tumor-specific marker for the diagnosis of hepatocellular carcinoma and has been widely used in clinical setting[21-25]. PCNA, the auxiliary protein of DNA polymerase δ, ε, plays an important role in DNA replication, repair and cell cycle control, and can be used as differentiation marker of hepatocarcinoma[26-30]. The increased levels of α-FP and PCNA are correlated with hepatocyte malignancy, and many differentiation inducers such as HMBA, retinoic acid and sodium butyrate could decrease their levels in hepatocarcinoma cells[18-20]. γ-GT and TAT also can be used as tumor markers of hepatocellular carcinomaIn[31-33]. The decreased activity of TAT and the increased levels of α-FP, PCNA and γ-GT activities are correlated with hepatocyte malignancy. Many differentiation inducers, such as retinoic acid and HMBA, could reduce the levels of α-FP and PCNA and γ-GT while increase the level of TAT in hepatocarcinoma cells[18,19,34]. Our results revealed that the levels of α-FP and PCNA and γ-GT were down-regulated and the activity of TAT was increased in the tachyplesin-treated cells, indicating that tachyplesin could alter the activities of differentiation-associated enzymes and decrease the levels of tumor-associated antigens as many differentiation inducers.
In addition, many tumor suppressor genes and oncogenes, which were correlative with the tumorigenesis and progression and prognosis of hepatocarcinoma, also could serve as index of induced differentiation. p21WAF1/CIP1, the pioneer member of p21 family of cyclin-cdk inhibitor class of proteins, has been implicated as a growth arrest mediator in cell terminal differentiation and apoptosis[1,30,35]. Many inducers such as sodium butyrate could increase the expression of p21WAF1/CIP1 of hepatocarcinoma cells[20]. In the meantime, c-myc gene also plays an essential role in cell proliferation, differentiation, and apoptosis[36,37]. Down-regulation of c-myc expression induced by differentiation signals is regarded as a hallmark of cell differentiation[38-40]. The expression of c-myc level was reduced in sodium butyrate-induced differentiation of human hepatoma cells[41], and in all-trans retinoic acid-induced differentiation of human leukemic U937 cells with an increase in p21WAF1/CIP1 expression[42]. Our results showed that tachyplesin could increase the expression of p21WAF1/CIP1 and down-regulate the levels of c-myc protein in SMMC-7721 cells, and induce the cells to terminal differentiation as other differentiation inducers[42,43].
Taken together, the results of the current study indicated that tachyplesin could effectively inhibit the proliferation of human hepatoma SMMC-7721 cells, reverse the malignant morphological and ultrastructural characteristics, alter the activities of differentiation-associated enzymes and the levels of tumor-associated antigens, adjust the expression of oncogenes and tumor suppressor gene correlative with hepatocellular carcinoma, and so induce hepatocarcinoma cell differentiation.