INTRODUCTION
For years, gallstone has been nearly the most common illness in digestive system all over the world, especially in China[1-9], and there are many methods to treat this illness[10-16], including many Chinese traditional medicines. Although some therapies were successful in the end, the best way to deal with the illness is to prevent it before it occurs. So it is important to clarify the key factors that promote the formation of gallstone. Although there were lots of researches in this field that intended to discover the secrets behind the gallstone[17-41], there are still lots of phenomena we cannot explain now.
For the mechanism of formation of pigment gallstone, the earliest suggestion came from Maki[42]. He indicated that bacterial infection induced the hydrolysis of conjugated bilirubin and increased the level of free bilirubin, which was the critical factor for gallstone formation. However, there are many cases of gallstone without bacterial infection. Moreover, the increase of bilirubin concentration is only an essential condition for the precipitation of bilirubin, but not enough to form stone.
In 1982, Elek et al[43] reported the ESR signal of pigment gallstone. Its intensity varied linearly with quantity of bilirubin. It gave a hint that the formation of pigment gallstone was likely to be linked with free radicals. Recently, a lot of facts indicate that free radicals are the triggers or important links of many diseases. They are relative to cell damages and mutation. In view of the relationship of pigment gallstone with inflammation and accompanying damages of liver, kidney, gastrointestinal system, probably there are certain carriers of free radicals in the circulation and the free radicals cause cell damages. Based on the Elek’s experiment, the carrier might be bilirubin. However, we have to illustrate:
Firstly, the attackers during inflammation are superoxide free radicals and hydroxyl radicals, then, how we can link the formation of pigment gallstone to these free radicals.
Secondly, whether or not bilirubin free radicals formed in situ during inflammation can induce cell damages and initiate the following pathological processes.
In this report, we demonstrate that bilirubin free radicals can be formed under the attack of other free radicals and induce evident cell damages. Then, the relationship between the formation of pigment gallstone and bilirubin free radicals will be discussed.
FORMATION OF BILIRUBIN FREE RADICAL AND ITS CHEMICAL NATURE
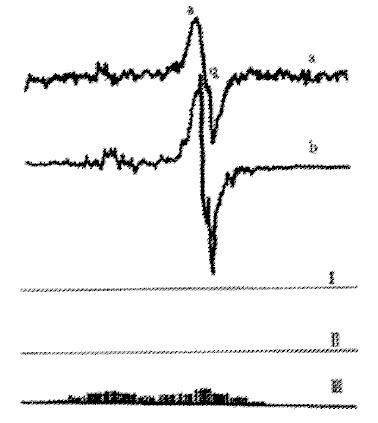
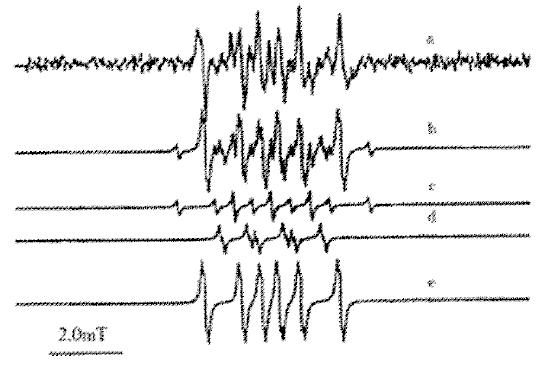
As we find, solid bilirubin absorbs oxygen reversibly when exposed to air, and meanwhile bilirubin free radicals are detectable by ESR[44]. ESR spectra showed that the free radicals signals of solid bilirubin were composed of a semiquinone signal and a superoxide radical signal (Figure 1). The former may be formed from the carbonyl group, while the latter may be bound with a metal ion, especially the iron that might be released from the haem from which bilirubin was produced. High spin Fe (II) or Fe (III) was found to be coordinated with four tetrapyrrol nitrogens, which was the same as in haem. Various free radical sources, such as FeSO4 + EDTA, XO/XOD and 60Co-irradiation were used to generate bilirubin free radicals. The ESR signals obtained were in accordance with those of natural pigment gallstone[45] (Figure 2). Moreover, the ESR signals of bilirubin became diminished after treatment with free radicals scavengers, such as SOD, mannitol and vitamin C, or ligand of Fe2+ or Fe3+[46].
Figure 1 Simulation of ESR spectrum of bilirubin.
(a) The experimental spectrum; (b): Simulating spectrum. (I) Superoxide radical; (II) Semiquinone radical; (III) Free electron of superoxideradical delocalized to tetrapyrrole (heterotropism is ignored).
Figure 2 ESR spectra of bilirubin treated with: (a) Bilirubin (from Sigma) as control; (b) 60Co (100Gy); (c) ·OH (Fe (II) + EDTA); (d) O2·- (XO/XOD).
The relative intensity of ESR peak is: a:b:c:d = 1:2.1:2.8:3.9
The above results support a mechanism suggested by Foote for photoxidation of bilirubin[47]: (where BR refers to bilirubin, and T1 represents the transition state of the reaction)
So (BR) hv T1 (BR) O2 BR++O2·- [BR+ + O2·-] [BR + O2] products
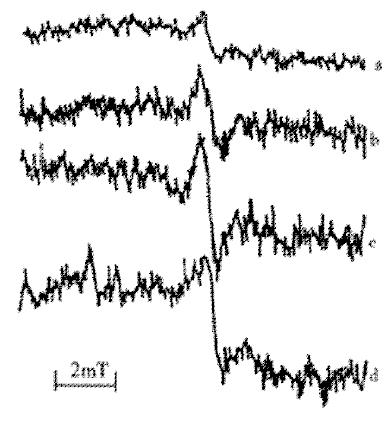
Bilirubin free radicals in solution were showed to be more complex than solid bilirubin free radicals. According to the ESR signals and computer simulation, it was deduced that the signals were composed of three groups of free radicals signals: ·H, O2·-, RCH2· (Figure 3)[48]. O2·- was from natural bilirubin, and the other two free radicals might be generated during attack of O2·- to C-C bond. In some experiments, only ·OH was trapped by DMPO, which was considered as the dismutation product of O2·-. In experiment at 77 K, semiquinone signal also could be identified just as in solid bilirubin.
Figure 3 ESR spectra of spin-trapping of bilirubin free radical in solution.
(a) DMPO-traping spectrum of bilirubin; (b) computer simulating spectrum; (c) Simulating spectru of DMPO-H; (d) Simulating spectrum of DMPO-OOH; (e) simulating spectrum of DMPO-CH2R.
In conclusion, we proved that bilirubin free radicals consisted of at least semiquinone free radicals and metal bound superoxide free radicals. In solution, O2·- attacks bilirubin to generate ·H and RCH2·, and also dismutates into ·OH. Because of the chemical properties of bilirubin free radical, its contributions to the formation of gallstone and its effects on cells discussed below become easier to understand.
PROPERTIES OF BILIRUBIN FREE RADICAL RELEVANT TO FORMATION OF PIGMENT GALLSTONE
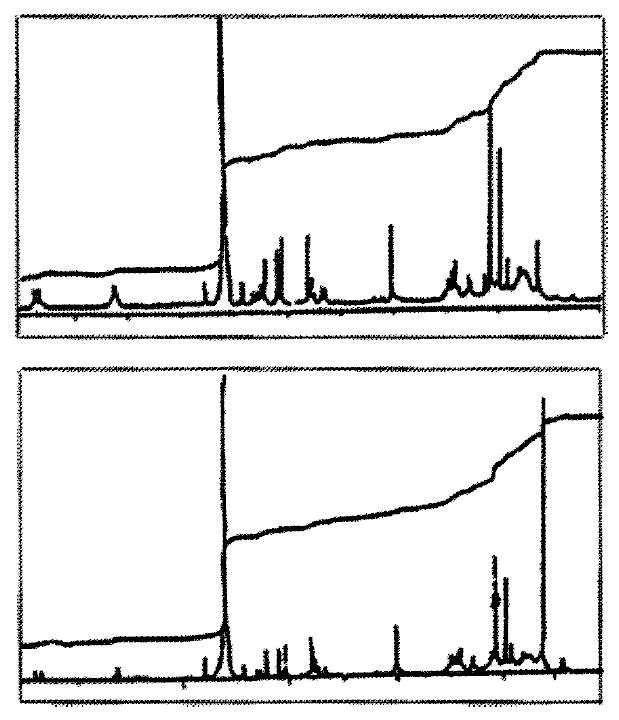
To explore the polymerization of bilirubin induced by free radicals, IR and NMR were used to compare the polymerization of original bilirubin or bilirubin treated with free radicals sources. The only significant variation in IR spectra was the decrease of the absorbance at 990 cm-1 (vinyl group). If the absorbance at 1610 cm-1 (carboxyl group) was taken as the inner reference[49], the ratio A990/A1610 was found to be 0.6470, 0.5646 and 0.5587 in untreated bilirubin and bilirubin treated with FeSO4 + EDTA and 60Co irradiation respectively. In NMR spectra, increase of the integral area of the methyl group (1.237 ppm) and decrease of that of the vinyl group (above 5 ppm) were observed (Figure 4).
Figure 4 IH-NMR spectra of bilirubin.
(A) commercial bilirubin (from Sigma); (B) bilirubin treated with 60Co.
Thus, we postulated that bilirubin molecules polymerized through the reaction between the vinyl group and the hydroxyl group by free radicals attack. This hypothesis was consistent with William’s suggestion[50].
By means of the light scattering method, the average molecular weight of the bilirubin free radicals in DMSO solution was determined in the range 60000-80000, which was higher than that of original samples (< 20000). The particle size distribution was measured by means of Coulter counter and the result showed that bilirubin free radicals became larger[51]. A kinetic study showed that the treated bilirubin reacted with calcium ion more rapidly than the untreated sample, and the conditional solubility product was found to be lower. These results suggested that bilirubin free radicals tended to polymerize and deposit, leading to the formation of gallstone.
Based on the above results, we considered that during the gallstone formation bilirubin reacted with the active-oxygen species formed in vivo and was changed into free radicals, then polymerized, aggregated and calcified. This might be an important step of formation of pigment gallstone.
CYTOTOXICITY OF BILIRUBIN FREE RADICAL AND ITS CONTRIBUTION TO THE FORMATION OF PIGMENT GALLSTONE
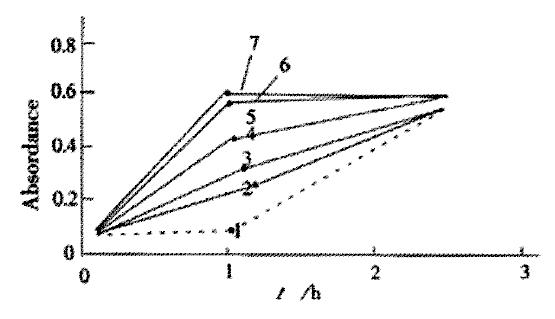
It is well known that bilirubin is cytotoxic. In our experiments, we found that bilirubin free radicals could induce phospholipid peroxidation (LPO), lactate dehydrogenase (LDH) leakage from hepatocytes (Figure 5), and the decrease of intracellular total glutathione (GSH) and oxidized glutathione (GSSG) levels (Figure 6)[52].
Figure 5 Leakage of lactate dehydrogenase (LDH) of hepa tocyte.
1. Control; 2-7. Treated with BRVC, BRcomm + BSA, BRcomm, BRCo, BRFe, BRXO/XOD
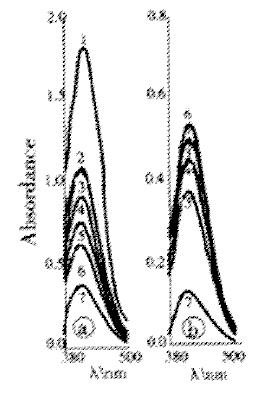
Figure 6 Absorption curve of total and oxidezed glutathione level of hepatocyte.
(A) Total glutathione; (B) Oxidized glutathione 1. Control; 2-7. Treated with BRVC, BRcomm + BSA, BRcomm, BRCo, BRFe, BRXO/XOD
The above effects can be diminished when hepatocytes were incubated with bilirubin treated with free radicals scavenger. So cytotoxicity of bilirubin might come from its chemical nature-free radical, just as what had been discussed above. In order to clarify the mechanism of bilirubin-induced cytotoxicity, we investigated effects of bilirubin free radicals on erythrocyte membrane. The SDS-PAGE results showed that after treatment of membrane with bilirubin free radicals, the integral area of band 1 and 2 decreased and some small molecular bands appeared between band 2 and 3 (Figure 7), which indicated that a part of membrane bound proteins, especially the spectrin, were degraded, then the membrane structure might be damaged. The above result was also supported by means of labeling membrane protein with fluorescamine (Figure 8)[53]. In addition, due to the degradation of membrane bound proteins, the increase of lateral movements of phospholipids, decrease of polarizability as well as decrease of micro-viscosity of erythrocyte membrane were observed by means of fluorescence polarization measurement[54]. The studies on the reaction between membrane and bilirubin free radicals showed that the process comprised three steps: firstly, a rapid formation of an electrostatic complex between bilirubin free radicals and polar groups of phospholipid, then a slow inclusion of bilirubin into hydrophobic core of membrane, and finally an erythrocyte membrane-induced bilirubin aggregation[55].
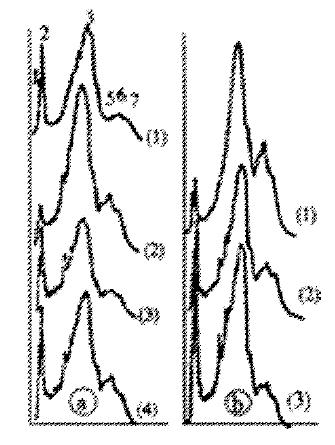
Figure 7 Scans of SDS-PAGE stained with Coomassie blue R-250.
(A) 1. control, 2-4 human erythrocyte membrane treated with BRCo, the irradation doses are 100, 50, 5 (Gy) respectively; (B) human erythrocyte membrane treated with BRFe, the concentrations of 1-3 are 16.67, 13.3, 6.7 (mmol/L) respectively
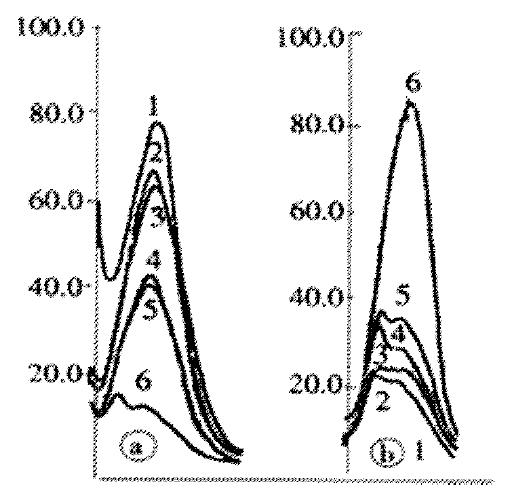
Figure 8 Fluorescence spectrum of erythocyte proteins labeled with fluorescamine.
(A) In the precipitated proteins; (B) In the supernatants. 1. control, 2.BRVC, 3.BRcomm, 4-5. BRFeªª (10 and 20 mmol/L) 6. BRCo (100 Gy)
In summary, bilirubin free radicals can damage the liver cells, which can induce the change of ingredients of the bile, decrease the amount of bile acid. Meanwhile, the abnormal metabolism in hepatocyte can lead to hydrolysis of the conjugated bilirubin, increase the concentration of free bilirubin, thus make bilirubin supersaturated to the bile and promote the formation of pigment gallstone. Moreover, the cell damages caused by free radicals can also promote excretion of glucoprotein, which might act as adhesives and increase the particle size of calcium bilirubinate.
CONCLUTIONS
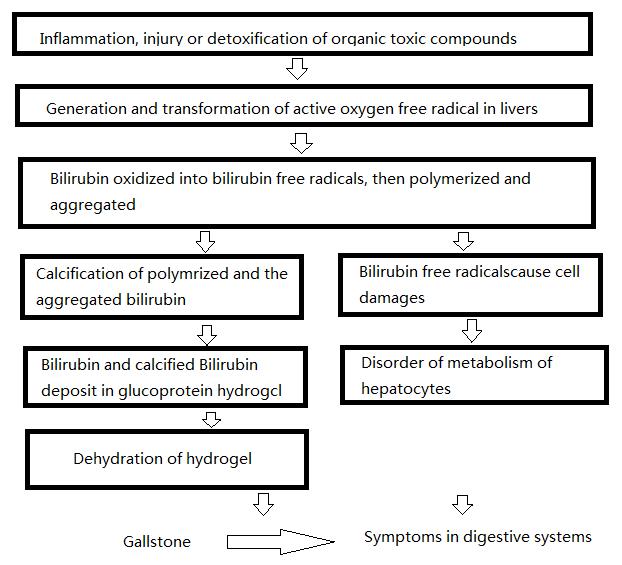
Based on our results, we considered that there were two ways by which bilirubin free radicals promoted the formation of pigment gallstone. On the one hand, due to the properties of free radicals, bilirubin free radicals formed in vivo were more liable to polymerize and aggregate, then induced the formation of stone. On the other hand, the damages on hepatocytes induced by bilirubin free radicals also impaired the cell function, then led to the disorder of metabolism, which gave rise to the formation of stone indirectly, as well as symptoms in digestive systems. The whole process is illustrated as follows:
Math 1