INTRODUCTION
Hereditary nonpolyposis colorectal cancer, or Lynch syndrome, is an autosomal, dominantly inherited disease characterized by an exess of early age onset colorectal cancers. It is estimated that hereditary nonpolyposis colorectal cancer may account for 5% to 10% of the total colorectal cancers worldwide[2]. There are two main variants of the disorder[1,3], Lynch syndrome I, which is susceptible to colorectal carcinoma only, and the more common Lynch syndrome II, which is characterized by excessive colorectal carcinoma and malignancies of extracolonic tissue, especially the endometrium in the western world. Other tumors belong to the spectrum of this syndrome include those of the stomach, esophagus, ovaries, pancreas, and urinary tract[3,4]. HNPCC has no premonitory symptoms, and the cancer is usually found at an advanced stage at around 50 years of age.
Five causative mismatch repair genes for HNPCC (hMSH2, hMLH1, hPMS1, hPSM2, and hMSH6/GTBP) have been identified, and a germline mutation in one of them predisposes carriers to early onset cancers[5-10]. hMSH2 and hMLH1 genes contribute at least 40% and 35% of all HNPCC germline mutations, respectively. Mutational analysis of the two main genes not only permit diagnosis of most cancer families or patients, but also earlier detection of cancer through targeted surveillance and chemoprevention of the gene carriers.
Despite the fact that China might have the biggest HNPCC population, the disease is rare reported. And also the mismatch repair gene mutation of HNPCC families from Mainland China has not been reported. We retrospectively reviewed the clinical characteristics and treatments of 16 HNPCC kindreds registered in our hospital, and report the first hMLH1 gene mutation family in Mainland China.
MATERIALS AND METHODS
Diagnotic criteria of HNPCC family
Kindreds of independent Chinese families that included multiple patients with colorectal cancer were clinically reviewed. The strict criteria for HNPCC were the Amsterdam criteria[11]. The criteria are (1) three or more relatives with histologically verified colorectal cancer, with one of them being a first-degree relative to the other two relatives; (2) at least two successive generations were affected; (3) one or more colorectal cancer cases diagnosed under 50 years of age; and (4) familial polyposis of colon is excluded. Japanese clinical diagnotic criteria for HNPCC[12] was used for highly suspected families that did not fully met the Amsterdam criteria. Families that met either A or B were clinically diagnosed as HNPCC. The criteria include A: A case with three or more colorectal cancers within the first-degree relatives; B: A case with two or more colorectal cancers within the first-degree relatives meeting the following criteria (1)age onset of colorectal cancers being earlier than 50 years old; (2) with right colon involvement; (3) with synchronous or metachronous multiple colorectal cancers and (4) associated with synchronous or metachronous extracolonic malignancies.
Family investigation
The diagnosis is made upon the compilation of a detailed family history. The family history of cancer was thoroughly reviewed when a patient was admitted with the diagnosis of colorectal cancer. Suspected families were further reviewed by a specific surgeon (Wang ZJ). The history covered at least all first-degree relatives and selected second-degree relatives. Families fulfilling the Amsterdam criteria or Japanese clinical diagnotic criteria were registered and family members were reviewed by telephone or outpatients visits. Before this review, all patients were reinvestigated by follow-up records or telephone interviews.Data concerning the site of cancer, age of diagnosis, history of synchronous and/or metachronous cancers, and histopathology of cancers were documented. The Clinical characteristics, diagnosis, treatments and follow-up study of sixteen HNPCC pedigrees were retrospectively analyzed in this report.
Germline hMLH1 gene mutation analysis
Peripheral blood was obtained from the affected and unaffected normal family members, all of whom signed the formal consent. Genomic DNA was extracted by standard organic methods. The coding regions of hMLH1 and hMSH2 gene were PCR amplified according to Weber methods[13] with minor modifications. SSCP was used to screen the cording regions and variant bands were then sequenced by a 377DNA sequencer. After a mutation was found, specific regions of DNA from their offspring were PCR amplified and sequenced.
RESULTS
HNPCC families
A total sixteen kindreds were diagnosed, among there, eleven met Amsterdam criteria and five met the Japanese clinical diagnosis criteria. There were three Lynch syndrome I families, and thirteen Lynch syndrome II families respectively. HNPCC constituted about 5.2% of colorectal cancer patients within the same period in our hospital.
Tumor spectrum
One hundred and one malignant neoplasms were found in sixty-eight patients (multiple cancer in twenty-one) with a mean age of 50.8 years in sixteen kindreds, including fifty colonic, seventeen rectal, eleven gastric, seven endometrial, and four esophageal, two each skin, pancreatic, lung and cervical, one each breast, ovarian cancer, gastric leiomyosarcoma and brain glioblastoma, respectively.There were forty-six colorectal cancer patients (including sixteen metachronous colorectal cancers). In the present group, 67.6% patients and 66.3% cancers are of colorectal ones. Right-side colon cancers constitute 47.8% of total cancers and 74.6% of colorectal cancers. 34.8% of colorectal cancers were metachronous ones.
Patient treatment and follow-up
81.6% patients received radical operations; other patients received chemotherapy, irradiation and traditional Chinese medicine treatment. 39.5% colorectal cancer patients had metachronous colorectal cancers within 10 years and required re-operations. A multiple primary cancer patient, after rectal cancer was resected, developed subsequently ascending colon cancer 12 years later, stomach cancer 18 years later, transverse colon cancer 19 years later, and descending colon cancer 28 years after the first operation.
hMLH1 mutation RESULTS
In one large HNPCC family, a germline G265T nonsense mutation was found in the third exon of hMLH1 gene. The mutation turned GAG in codon 89 to a stop codon TAG, resulting in a truncated protein. The normal family member over 50 years old did not have the same germline mutation. Three phenotypically normal family members were also found to carry the mutated gene. Two of them subsequently received colonoscopy examinations. One was found to have an adenoma of 2 cm and was resected endoscopically. We searched the Human Gene Mutation Database (HGMD) (uwcm.web.cf.ac.uk/uwcm/mg/hgmdo.html), and found this mutation had not been reported previously in the literature.
DISCUSSION
Hereditary nonpolyposis colorectal cancer is a typical auto-dominant hereditary disease[14], constituting about 5.2% of colorectal cancer patients within the same period in our hospital, which is consistent with the published reports[14,15]. Early onset of the colorectal cancer is an important feature of HNPCC patients[16]. The average age onset of colon cancer in the general population in China is around 60 years of age. Patients with HNPCC in our group developed colorectal cancer at an average of 49 years, 10 years earlier than the general population. Colorectal cancers occur most frequently in HNPCC kindred. In the present group, 67.6% patients and 66.3% cancers are colorectal ones, consistent with previously published reports[17,18]. Colon cancers are more often right-sided, constituting 47.8% of total cancer and 74.6% of colorectal cancers, whereas in sporadic colon cancer, the opposite is true[17,18]. The next most frequently seen cancer is gastric cancer, about 10.8% in this group, well above the reported frequency in the literature[19,20]. It is interesting that gastric cancer occurred at a higher frequency and ovarian cancer at a lower frequency in our group[21,22]. More research study should be done to clarify if this is just a reflection of case selection bias or the difference is related to ethnic or geographical groups[23,24]. Endometrium is the third predisposing organ, and about 6.9% cancer is in the endometrium[21]. A small number of other cancers included pancreatic, esophageal, skin, lung, cervical, and breast cancers. There was one gastric leiomyosarcoma and glioblastoma, respectively.
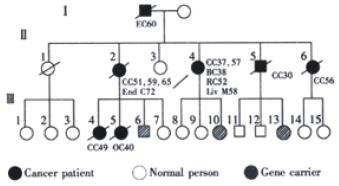
Two breast and two lung cancers in our small group of patients are worthy of particular comment. Controversy exists whether these two kinds of cancers belong to the spectrum of HNPCC[25,26]. In China, breast and lung cancers are much less prevalent than those in the western worle, and it is interesting that the incidence of these two cancers among those families is far above the average level in China. One breast cancer occurred in the index patients of a Lynch syndrome II kindred (Figure 1) that received sequence analysis in this study. The patient developed ascending colon cancer at 37 years of age, breast cancer at 38, rectum cancer at 52, descending colon cancer at 57. The possibility of a sporadic breast cancer in this patient is small. The two lung cancers occurred in two patients of classical Lynch syndrome II kindred, but they developed rather late. More cases and studies are needed to clarify if these two cancers are integral to cancer spectrum of this syndrome[27].
Figure 1 A Lynch syndrome II pedigree with hMLH1 gene germline mutation.
CC: Colon Cancer; RC:Rectal Cancer; BC: Breast Cancer; OC: Ovarian Cancer; EC: Esophegeal Cancer; End C: Endometrial Cancer; Liv M: Liver Metastasis.
Another cardinal features of the HNPCC in this group and in the medical literature is the occurrence of metachronous colorectal cancer[28,29]. In our group, 39.5% colorectal cancer patients developed metachronous colorectal cancer within ten years after their initial colorectal cancer resection. But in contrast to the literature, none of the patients were found to have synchronous colorectal cancers. Although it is quite possible that small benign adenomas were not recorded or missed during the colonoscopic or barium examination, but the possibility of missing a cancer is quite small. The reason for the lack of synchronous cancer remains unclear.
81.6% our patients received routine radical resection. Among colorectal patients who received routine segmental resections, 39.5% developed metachronous colorectal cancer, and needed re-operations. After segmental resection, a multiple primary cancer patient developed ascending colon cancer twelve years later, gastric cancer eighteen years later, transverse colon cancer nineteen years later, and descending colon cancer twenty eight years later after the first operation. Therefore, the most eligible choice is subtotal or total colectomy with ileorectal anastomosis or with ileopouch anal anastomosis[30,31], which removes the colonic segment as much as possible, and avoids permanent ileostomy. Two patients, one had primary cancer, the other had metachronous colon cancer, both received total coloproctectomy with ileopounch anal anastomosis. A short term follow-up of the patients showed good life quality. In literature[30-33], different views exist regarding prophylactic coletomy for gene carriers of HNPCC. Firstly, not all carriers of the germline hMSH2 and hMLH1 gene mutations develop colorectal cancer; Vasen et al[32] reported that up to 60 years of age, 92% of the carriers developed colorectal cancers in affected families. Secondly, complications even mortality occurred around 8% and 1% respectively in these operations[30]. The timing of prophylactic operation in an HNPCC gene carrier is unknown. The median age at diagnosis of colorectal cancer is around 45 years, but Rodriguez-Bigas showed that 27% of their patients were diagnosed before 39 years and 88% by 69 years of age[33]. Other uncertainties include: the optimal procedure has yet to be considered, the psychologic impact of the invasive procedure on body image and sexuality in young adult carriers.
Colonoscopy remains the most important surveillant measure in revealing synchronous and metachronous cancers or polyps in the follow-up of patients and in symptomatic gene-carriers. polypectomy should performed if present[34]. J vinen et al[35] reported a 62% decrease of colorectal cancer in HNPCC patients who underwent colonoscopic screening vs those who did not.
The first family with hMLH1 gene mutation from Mainland China was identified in our study. In the large HNPCC kindred, a germline G265T nonsense mutation was found in the third exon of hMLH1, resulting in a stop codon and truncated protein. The mutation segregated with the disease in the family. We made a search in the Human Gene Mutation Database (HGMD) (uwcm.web.cf.ac.uk/uwcm/mg/hgmdo. html), and found this to be a new mutation previously unreported in the medical literature. In three phenotypical normal family members, the same mutated gene was found in the germline. Two of them subsequently received colonoscopy examinations, and one was found to have an adenoma of 2 cm which was resected endoscopically. These carriers are now needed an intensive follow-up regime.