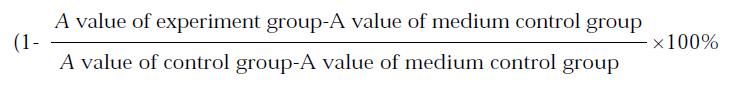Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.87
Revised: December 11, 2001
Accepted: December 29, 2001
Published online: February 15, 2002
AIM: To observe the biological specialization of human peripheral blood dendritic cells (DC) and cord blood derived DC and its effects on effector cells killing human hepatocarcinoma cell line BEL-7402 in vitro.
METHODS: The DC biological characteristics were detected with immunohistochemical and MTT assay. Two antitumor experimental groups are: peripheral blood DC and cord blood DC groups. Peripheral blood DC groups used LAK cells as the effector cells and BEL-7402 as target cells, while cord blood DC groups used CTL induced by tumor antigen twice pulsed DC as effector cells and BEL-7402 as target cells, additional peripheral blood DC and cord blood DC are added to observe its stimulating activities to effector cells. The effector¡äs cytotoxicity to tumor cells were detected with neutral red colorimetric assay at two effector/ target ratios of 5:1 and 10:1.
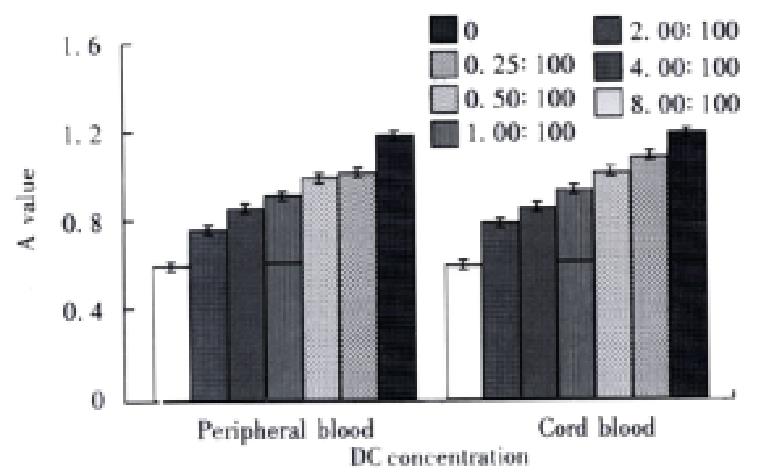
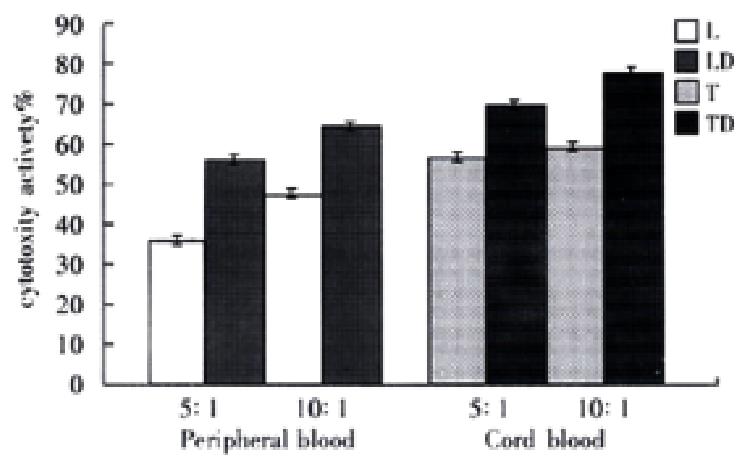
RESULTS: Peripheral blood DC and cord blood DC highly expressed HLA-ABC, HLA-DR, HLA-DQ, CD54 and S-100 protein. The stimulating activities to lymphocyte proliferation were compared between experimental groups (DC added) and control group (no DC added), in six experiment subgroups,the DC/lymphocyte ratio was sequentially 0.25:100, 0.5:100, 1:100, 2:100, 4:100 and 8:100, A values(-x±s) were 0.75396 ± 0.009, 0.84916 ± 0.010, 0.90894 ± 0.012, 0.98371 ± 0.007, 1.01299 ± 0.006 and 1.20384 ± 0.006 in peripheral blood DC groups and 0.77650 ± 0.005, 0.83008 ± 0.007, 0. 92725 ± 0.007, 1.05990 ± 0.010, 1.15583 ± 0.011, 1. 22983 ± 0.011 in cord blood DC groups. A value was 0.59517 ± 0.005 in control group. The stimulating activities were higher in experimental groups than in control group (P < 0.01), which were increased when the DC concentration was enlarged (P < 0.01). Two differently derived DCs had the same phenotypes and similar stimulating activities (P > 0.05). In peripheral blood DC groups, the cytotoxicity (-x±s) of the LD groups (experimental groups) and L groups (control group) was 58.16% ± 2.03% (5:1), 46c18% ± 2.25% (10:1) and 38c13% ± 1.29% (5:1) and 65.40% ± 1.56% (10:1) respectively; in cord blood DC groups, TD groups (experimental groups) and T groups (control groups) were 69.71% ± 2.33% (5:1), 77.64% ± 1.94% (10:1) and 56.89% ± 1.82% (5:1) and 60.99% ± 1.42% (10:1) respectively. The cytotoxicity activities were enhanced with increased effector/target ratio (P < 0.01). At the same effector/target ratio, the cytotoxicity of experimental groups were bigger than that of control groups (P < 0.01). The cytotoxicity activities of cord blood DC groups were higher than that of peripheral blood DC groups (P < 0.01).
CONCLUSION: Peripheral blood DC and cord blood DC are mature DC in morphology and function, both can enhance the effector cell killing activities to hepatocarcinoma cells. DC pulsed with tumor antigen can induce higher specific CTL activity than unpulsed DC.
- Citation: Zhang JK, Li J, Chen HB, Sun JL, Qu YJ, Lu JJ. Antitumor activities of human dendritic cells derived from peripheral and cord blood. World J Gastroenterol 2002; 8(1): 87-90
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/87.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.87
Dendritic cells (DC) is a potent professional antigen presenting cell, the only one that can stimulate the naive T cell[1-4]. DC can present exogenous antigen to CD4+ cell by MHC-II antigen presenting pathways as well as to CD8+ cell by MHC-I pathways. It also provides plenty of costimulating signals, so that it plays a key role in antitumor immunity[5-9]. Although the peripheral blood DC is easily separated, DC weas able to enhance the killing activity of Lymphkine and PHA activated killer (LAK) cells in vitro[10-12], but in some patients with tumors, especially some patients with advanced tumors, autogenous DC may be defective.In this article, two differently derived DCs are studied on their induction of anti-hepatocarcinoma cell activity. It provides experimental evidence for clinical application of DC directed tumor immunotherapy.
Human peripheral blood provided by young volunteers, and cord blood provided by Shantou University MedicalMollege FirstAffiliated Hospital.
BEL-7402 tumor cell line was bought from Experimental Animal Center,Sun Yat-Sen University of Medical Sciences.
Percoll was purchased from Pharmacia.Mini-MACS(magnetic activated cell sorter) and CD34 cell separation kit were purchased from Miltenyi GmbH Biotec, a kit including the following reagents: A1-human Ig (FcR), A2-haptin coupled CD34 monoclonal antibody, B-colloid anti-haptin antibody and microbead. rhSCF, rhGM-CSF and rh TNF-αwere obtained from Pepro Tech Ltd or Institute of Basic Medicine Sciences,Chinese Military Medical Academy.Mouse anti-human antibody CD54, HLA-ABC, HLA-DR, HLA-DQ, S-100 protein and SABC immunohistochemical kit were obtained from Biotec, Boehringer Mannheim and Boster, respectively. MTT was from Amresco.
Isolation of Human Peripheral blood DC[9] Four step method of our laboratory was used.Peripheral blood mononuclear cells from healthy volunteers were prepared using Ficoll-Hypaque (ρ = 1077 g·L-1) centrifugation method. Interface cells were collected and washed three times to remove platelets. Discontinuous Percoll density gradient centrifugation was employed, and interface cells between 35% and 50% called preliminary enrichment of DC were collected, cultured in PRMI 1640 with 100 mL·L-1 inactivated fetal calf serum (100 mL·L-1 FCS PRMI 1640) at 37 °C, in a saturation humidity, atmosphere of 50 mL·L-1 CO2 for 36 hours, then panned on Ig coated petri dish for further purification, nonadhesive cells were collected as the mature DC.
Isolation of human cord blood DC The CD34+ stem cells, were seperated using CD34+ stem cell separation kit and microbead, Mini-MACS cell sorter, cultured with rhGM-CSF, rhTNF-α and rhSCF for 14 d, mature DC was acquired.
Peripheral blood DC smear and cord blood DC smear were prepared and incubated with mouse anti human HLA-ABC, CD54, HLA-DQ, HLA-DR and S-100 protein primary antibody. ABC staining and DAB were used to display the result.
DC stimulating activity to homogenous lymphocyte proliferation Human peripheral blood lymphocytes were obtained by Ficoll separation method. Two groups of peripheral blood DC and cord blood DC were divided. In each group, six subgroups were divided according to the DC/lymphocyte ratio of 0.25:100, 0.5:100, 1:100, 2:100, 4:100 and 8:100 respectively. Lymphocyte concentration was 8 × 108·L-1, PHA was 50 mg·L-1. Control group as DC+PHA served as control in each subgroup. Additional lymphocyte + PHA and PHA also served as control groups. Each subgroup set three wells on 96 multiwell culture plates. Each experiment repeated 4 times.
MTT colorimetric method detecting the lymphocyte proliferation Add 20 μL MTT (5 g·L-1) to each well of multiwell culture plate, incubate for 4 h, then add 150 μL DMSO, mixed about 10 min until the crystal completely dissolved. The absorption value (A value) of each well was immediately read by Bio-Rad 3550-UV type automatic enzyme linked detector at 490 nm wavelength. The minus of A value in experimental group and A in DC + PHA shows the proliferative response. The minus of A value in lymphocyte + PHA group and A in PHA shows the lymphocyte proliferation of control group. SPSS software was applied for analysis of variation.
LAK cell induced The human peripheral blood mononuclear cells were prepared by the same procedure above, cultured at 2 × 109·L-1 population with the final concentration of rhIL-2 1000 kU·L-1 and PHA 20 mg·L-1 in 100 mL·L-1 FCS PRMI-1640 at 37 °C in a full humidified 50 mL·L-1 CO2 atmosphere for 7 d. Half volume of solution was replaced by fresh culture medium at d4.
CTL induced twice by antigen pulsed DC The whole culture system included human peripheral mononuclear cells 1 × 108·L-1, cord blood DC 5 × 106·L-1, ultrasonic disrupted BEL-7402 cells 1 × 109·L-1, IL-2 80 kU·L-1. They were cultured for 5 d and pulsed again at d3. Control culture system (no DC added) was set.
DC induced CTL killing activity to hepatocarcinoma cells The experiment was conducted two groups: peripheral blood DC group and cord blood DC group, each group being divided into two subgroups. Peripheral blood DC groups: BEL-7402 + LAK (L group) as control group, BEL-7402 + LAK + DC (LD group) as experiment group. BEL-7402 cell concentration was 8 × 108·L-1, DC was 8 × 106·L-1, two LAK /BEL-7402 ratio of 5:1 and 10:1 were applied.Cord blood DC groups: BEL7402 + CTL (T group) as control group, BEL7402 + DC-CTL (TD group) as experimental group. Cell concentration and ratio were the same as above. Additional BEL- 7402 culture media was set as control group. Each group set three paralleled wells, cultured in 96 multiwell culture plate for 48 hours, the effetor cell killing activities were detected. The procedure above was repeated for 4 times.
Neutral red uptake method Neutral red uptake method was applied to detect the cytotoxicity activities of effetor cells,0.1 mL neutral red solution 0.3 g·L-1 was added to each well, incubated at 37¡æ for 1 h, rinsed with PBS, solution of hydrochloride ethanol 0.1 mL was added, and absorbance was detected at 570 nm by Bio-Rad automatic enzyme linked detector. Formula for cytotoxicity calculation is below:
Math 1
SPSS for windows statistic software are used for data variation analysis.
Immunohistochemical ABC method showed that human peripheral blood DC and human cord blood DC had high expression of HLA-ABC, HLA-DR, HLA-DQ and CD54. S-100 protein was also positive. Positive cells were big and irregular in shaped and filled with diffuse brown-yellow particles in cytoplasm, the neucleus was also big and irregular. However, the phenotype difference between peripheral blood DC and cord blood DC was not distinct.
In human peripheral blood DC and human cord blood DC groups, lymphocyte proliferation activities were significantly higher than control groups (P < 0.01), which was increased when DC concentration was enlarged (P < 0.01). Human peripheral blood DC and human cord blood derived DC had no significant difference in lymphocyte stimulation (P > 0.05, Figure 1).
In groups of human peripheral blood DC and human cord blood DC, cytotoxicity activities enhanced with the increased effector/ target ratio (P < 0.01). Within the same ratio, cytotoxicity activities of experimental groups were higher than control groups (P < 0.01). Cytotoxicity activities of human cord blood DC groups were bigger than human peripheral blood DC groups (P < 0.01, Figure 2).
DC is a potent antigen presenting cells, mainly takes part in cell immunity and T cell dependent humoral immunity, and plays a key role in antitumor immunity[13-21]. Recently, with the construction of DC isolation method and expanding culture in vitro, research has transfer red from the relationship between tumor infiltrating DC and the prognosis to DC application in tumor immunotherapy, especially how to improve the tumor cell immunogenocity and enhance the DC antigen presenting efficacy and stimulating activity to CTL[22-30]. In this experiment, DC of human peripheral blood and cord blood were studied on its potential in clinical application.
Human peripheral blood DC isolation was made according to four step method modified in this laboratory. This method is easy to operate, low in cost and reliable, and has been used in this laboratory for many years, and high purity of DC can be obtained by this method[31,32]. Another method is used in cord blood DC isolation: CD34+ cell isolation kit combined with cell factor expanding culture for preparation of cord blood derived DC. This is an advancing method. The principle of CD34+ cell isolation kit is as follows: CD34+ monoclonal antibody recognizes the specific antigen of stem cell membrane, by which the antibody coupled magnetic microbead binds to cells, when the cells pass through column in the magnetic field, the CD34+ can be acquired by positive selection. Three reagents comprises in CD34+ isolation kit: A1, human Ig, used as blocking reagent to FcR for preventive non specific binding of CD34+ monoclonal antibody to CD34- cells. A2, hapten coupled CD34 monoclonal antibody, can specifically bind with CD34 molecule. B, anti-hapten antibody linked with microbead, can link the microbead with CD34+ cells. When the cells pass through MACS (magnetic cell sorter) column in magnetic field, negative cells can pass through the column, while positive cells were absorbed to column. When the column was token away from magnetic field, the elution from column included the positive cells. MACS cell isolation has been verified by immunofluorescent PCR, FISH and FACS method. It has the characteristics of high purity (93%-99.9%)[14], large number of cells processing ability in a single time, and easy operating, simple procedure. When cell factors such as rhGM-CSF, rhTNF-α and SCF are added to stem cell culture media, most of CD34+ cells differentiate to DC[33]. Though the GM-CSF can stimulate cell growth of both the DC progenitor and monocyte or macrophage, for high purity of CD34+ cell in initiate couture system, clearance most of monocyte and macrophage by its adherence to flask by replacing media and culture plate.Cell factor secreted by monocyte and macrophage also benefits DC development.
In this article, a series of antibodies were used for immunohistochemical staining of DC, results showed that human peripheral blood DC had and CD34+ derived cord blood DC high expression of CD54, HLA-ABC, HLA-DR, HLA-DQ, and S-100 protein.The positive cells accounted for above 95% and 90% respectively, demonstrating that DC here is mature[34,35].
For DC functional analysis, MTT assay was used to detect the DC activity of stimulating the allogenious lymphocyte.The principle of MTT assay is that the living proliferating cells can deoxidize the MTT (thiazoyl blue tetrazolium bromide) to purple crystal formazan and deposit in cytoplasm, so we can use the colorimetric method to detect the cell proliferation. With continuous modification, it has become a very consummate method with characteristics of sensitivity, simple procedure, safety and no radioactivity. In this experiment, DC can clearly stimulate the lymphocyte response to PHA. It shows that the DC has potent MLR stimulating activity which contributes to DC expression of adherence and MHC-II molecule. Phenotype and functionally mature DC of high purity provided primitive condition for DC application in antitumor.
Tumor cells expressed low level antigen and has antigen modulation, so tumor antigen can not be efficiently presented and the T cell mediated immune response can not be activated, by which tumor can escape the surveillance of immune system. As a nonprofessional APC, tumor cells with no expression of costimulator often leads to T cell anergy. Special attention has been paid to DC for its present exogenous antigen to CD8+ cell by MHC-I antigen presenting pathway as well as its expression of costimulating signal[36-40]. In this article, peripheral and human cord blood DC can significantly improve effector’s cytotoxicity, due to a large quantity of dendrites, and many kindsof surface molecules and receptors and cytokine secreted[41,42]. LAK cells induced for 7 days chiefly demonstrated CTL’s characteristic of CD16-, CD8+ and CD3+,which can efficiently kill the target cells[43-46]. It has been found recently that DC secretion of exosome can present antigen and induce immune response. This is another path for effector activation[47]. In general,from patients in well condition, autogenous peripheral blood DC and LAK cells can be acquired, for it is low in cost; while in patient in bad condition, cord blood DC can be used as an alternative.
Cord blood DC can more efficiently induce effector’s cytotoxicity than peripheral blood DC, due to the following factors: ⑴ Cord blood DC comprises some immature DC,the coexistence of mature and immature DC can be synergetic, immature DC can ingest and process antigen, while mature DC can present antigen and activate T cells, therefore, coexistence of mature and immature DC is better than single mature DC[48]. ⑵ Both cord DC and CTL were pulse twice with tumor antigen, and specific antitumor activity improved. LAK cells induced7 days can secrete perforin and granular particles nonspecific to ally kill target cells while human cord blood DC pulsed in vitro by tumor antigen can efficiently present tumor antigen to effector which occupy the TCR of CTL, and activate the specific CTL, with the help of costimulator such as CD80,CD86 and CD40. Furthermore, DC can secrete nave T specific chemotactic factor DC-CCK. Some other cell factors such as MCP-1, RANTES and IL-8 also can also play a chemotactic role in DC emigrant. DC can form a cluster of cells and secrete a large number of IL-12 which bind with IL-12R of CTL and enhance CTL proliferating response and cytotoxicity. IL-12 mediates TH1 immune response and inclines to tumor killing activity[42,49-51].If permitted, twice antigen pulsed DC should be used.
Summary, human blood DC and cord blood DC have a potential application in the clinical therapy of hepatocarcinoma, especially late hepatocarcinoma.
Edited by Ma JY
| 1. | Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1396] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 2. | Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66:205-208. [PubMed] |
| 3. | Bottomly K. T cells and dendritic cells get intimate. Science. 1999;283:1124-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cao X, Zhang W, Wang J, Zhang M, Huang X, Hamada H, Chen W. Therapy of established tumour with a hybrid cellular vaccine generated by using granulocyte-macrophage colony-stimulating factor genetically modified dendritic cells. Immunology. 1999;97:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Hu JY, Wang S, Zhu JG, Zhou GH, Sun QB. Expression of B7 costimulation molecules by colorectal cancer cells reducestumorigenicity and induces anti-tumor immunity. World J Gastroenterol. 1999;5:147-151. [PubMed] |
| 6. | De Veerman M, Heirman C, Van Meirvenne S, Devos S, Corthals J, Moser M, Thielemans K. Retrovirally transduced bone marrow-derived dendritic cells require CD4+ T cell help to elicit protective and therapeutic antitumor immunity. J Immunol. 1999;162:144-151. [PubMed] |
| 7. | Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1760] [Cited by in RCA: 1707] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 8. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10726] [Article Influence: 397.3] [Reference Citation Analysis (0)] |
| 9. | Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947-1953. [PubMed] |
| 10. | Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different time in killing hapatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673-675. |
| 11. | Sun JL, Zhang JK, Chen HB, Cheng JD, Qiu YQ. Promoting effects of dendritic cells on LPAK cells killing human hepatoma cells. Zhongguo Zhongliu Linchuang Yu Kangfu. 1998;5:16-18. |
| 12. | Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendritic cells against human hepatoma cell line. Shijie Huaren Xiaohua Zazhi. 1999;7:191-193. |
| 13. | Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520-4529. [PubMed] |
| 14. | Mackensen A, Krause T, Blum U, Uhrmeister P, Mertelsmann R, Lindemann A. Homing of intravenously and intralymphatically injected human dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Cancer Immunol Immunother. 1999;48:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Salgaller ML, Tjoa BA, Lodge PA, Ragde H, Kenny G, Boynton A, Murphy GP. Dendritic cell-based immunotherapy of prostate cancer. Crit Rev Immunol. 1998;18:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Lim SH, Bailey-Wood R. Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer. 1999;83:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Greten TF, Jaffee EM. Cancer vaccines. J Clin Oncol. 1999;17:1047-1060. [PubMed] |
| 18. | Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Wang RF. Human tumor antigens: implications for cancer vaccine development. J Mol Med (Berl). 1999;77:640-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti tumor immune response. Shijie Huaren Xiaohua Zazhi. 1999;7:161-163. |
| 21. | Li MS, Yuan AL, Zhang WD, Chen XQ, Tian XH, Piao YJ. Immune response induced by dendritic cells induce apoptosis and inhibit proliferation of tumor cells. Shijie Huaren Xiaohua Zazhi. 2000;8:56-58. |
| 22. | Xiao LF, Luo LQ, Zhou Y, Huang SL. Studt of the phenotype of PBLs activated by CD28/CD80 and CD2/CD58 and acting with hepatoma cells and the restricted usage of TCR V â gene subfamily. Shijie Huaren Xiaohua Zazhi. 1999;7:1044-1046. |
| 23. | Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother. 1998;46:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56-58. [PubMed] |
| 25. | Marriott I, Inscho EW, Bost KL. Extracellular uridine nucleotides initiate cytokine production by murine dendritic cells. Cell Immunol. 1999;195:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Sun JL, Zhang JK, Chen HB, Zhou YQ. Morphology of cultured human peripheral blood dendritic cells and their antitumor activity. Zhongguo Zuzhihuaxue yu Xibaohuaxue Zazhi. 1999;8:28-31. |
| 27. | Nunez R, Grob P, Baumann S, Zuniga A, Ackermann M, Suter M. Immortalized cell lines derived from mice lacking both type I and type II IFN receptors unify some functions of immature and mature dendritic cells. Immunol Cell Biol. 1999;77:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Wu MC. Progress in surgical treatment of primary hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:921-923. |
| 29. | Zou QY, Li RB, Zheng PL, Yang LP, Chen YZ, Kong XP. Effect of embryohepatic extracts on proliferation and differentiation of hepatoma BEL-7402 cells. Shijie Huaren Xiaohua Zazhi. 1999;7:243-245. |
| 30. | Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584-5591. [PubMed] |
| 31. | Zhang JK, Sun JL, Chen HB, Zeng Y, Qu YJ. Influence of granulocyte macrophage colony stimulating factor and tumor necrosis factor on anti-hepatoma activities of human dendritic cells. World J Gastroenterol. 2000;6:718-720. [PubMed] |
| 32. | Sun J, Zhang J, Chen J, Chen H, Chew Y, Chen J. In vitro study on the morphology of human blood dendritic cells and LPAK cells inducing apoptosis of the hepatoma cell line. Chin Med J (Engl). 2001;114:600-605. [PubMed] |
| 33. | Herlyn D, Birebent B. Advances in cancer vaccine development. Ann Med. 1999;31:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Austyn JM. Dendritic cells. Curr Opin Hematol. 1998;5:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Zhai SH, Liu JB, Zhu P, Wang YH. CD54,CD80,CD86 and HLA-ABC expressions in liver cirrhosis and hepatocarcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:292-295. |
| 36. | Reid SD, Penna G, Adorini L. The control of T cell responses by dendritic cell subsets. Curr Opin Immunol. 2000;12:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | MacPherson G, Kushnir N, Wykes M. Dendritic cells, B cells and the regulation of antibody synthesis. Immunol Rev. 1999;172:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Dubois B, Bridon JM, Fayette J, Barthélémy C, Banchereau J, Caux C, Brière F. Dendritic cells directly modulate B cell growth and differentiation. J Leukoc Biol. 1999;66:224-230. [PubMed] |
| 40. | Mailliard RB, Dallal RM, Son YI, Lotze MT. Dendritic cells promote T-cell survival or death depending upon their maturation state and presentation of antigen. Immunol Invest. 2000;29:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Stockwin LH, McGonagle D, Martin IG, Blair GE. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol Cell Biol. 2000;78:91-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Wykes M, MacPherson G. Dendritic cell-B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Huang SL, Xiao LF, Luo LQ, Chen HQ. Phenotype analysis and restricted usage of TCR Vâgenes subfamily in mAb-costimulated T cells after incubated with hepatocellular carcinoma cell line. Shijie Huaren Xiaohua Zazhi. 1998;6:1033-1035. |
| 44. | Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. Shijie Huaren Xiaohua Zazhi. 1998;6:1013-1016. |
| 45. | Chen Q, Ye YB, Chen Z. Activation of killer cells with soluble gastric cancer antigen combined with anti-CD(3) McAb. World J Gastroenterol. 1999;5:179-180. [PubMed] |
| 46. | Zhang JK, Sun JL, Chen HB, Zhou YQ. Ultrastructural comparison of apoptosis of human hepatoma cells and LAK cells. Shijie Huaren Xiaohua Zazhi. 1998;6:877-879. |
| 47. | Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1699] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 48. | Fujii S, Fujimoto K, Shimizu K, Ezaki T, Kawano F, Takatsuki K, Kawakita M, Matsuno K. Presentation of tumor antigens by phagocytic dendritic cell clusters generated from human CD34+ hematopoietic progenitor cells: induction of autologous cytotoxic T lymphocytes against leukemic cells in acute myelogenous leukemia patients. Cancer Res. 1999;59:2150-2158. [PubMed] |
| 49. | Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Rosenzwajg M, Camus S, Guigon M, Gluckman JC. The influence of interleukin (IL)-4, IL-13, and Flt3 ligand on human dendritic cell differentiation from cord blood CD34+ progenitor cells. Exp Hematol. 1998;26:63-72. [PubMed] |
| 51. | Höltl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |