Published online Dec 15, 2001. doi: 10.3748/wjg.v7.i6.884
Revised: April 1, 2001
Accepted: April 5, 2001
Published online: December 15, 2001
ATM: To describe a rapid technique for procurement of donor liver with aortic perfusion only (APO).
METHODS: Only the aorta is cannulated and perfused with chilled preservation solution.
RESULTS: The quality of donor liver can ensure the grafted liver functions.
CONCLUSION: The method of APO can simplify the operative procedure, compared with the dual cannulation. It also can minimize the danger of injuring vascular structures and involve less dissection.
- Citation: Lin QY, Chui K, Rao AN. Rapid donor liver procurement with only aortic perfusion. World J Gastroenterol 2001; 7(6): 884-886
- URL: https://www.wjgnet.com/1007-9327/full/v7/i6/884.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i6.884
A rapid technique of harvesting the donor liver helps to ensure the quality of the grafted liver. The aim of this report is to describe a simple and rapid method for procurement of the donor liver using aortic perfusion only.
At induction of anaesthesia, cefuroxime and methyprednisolone are given intravenously to the donor. A midline incision is made with electrocautery from the suprasternal notch to pubis. The sternum is spilt with a sternal saw, and hemostasis is obtained. Self-retaining retractors for the sternum and the abdominal wall provided excellent exposure. At this point the liver is evaluated, with particular attention paid to its consistency, color, and size. The liver is mobilized first by dividing the falciform ligament down to the suprahepatic vena cava. The left lateral section of the liver is mobilized by dividing the left triangular ligament and lesseromentum. At this stage it is important to check for a accessory left hepatic artery (HA) coming off the left gastric artery (LGA). The right colon and the caecum are mobilized together with the mesentery of the intestine to expose the aorta, superior menteric artery (SMA), inferior vena cava (IVC), left renal vein and inferior mesenteric vein (IMV). The IMV is ligated and divided. Both ureters are identified and slung with vascular loops. The first step is the dissection of the IVC from above its bifurcation to the left renal vein level, with a No. 1 silk encircled above the bifucation. A small segment of the aorta just above the bifurcation is cleared, and the No. 1 silk ties are looped at two sites above the bifurcation. The aorta is dissected to view the SMA. The inferior mesenteric artery (IMA) may be ligated and divided with 3-0 vicryl sutures to prevent loss of cold perfusion. The fundus of the gallbldder is incised and the bile is washed out with saline using a 50-mL bladder syringe. The duodenum is Kocherised then a search is made for an anomalous right hepatic artery arising from the SMA, which occasionally can be the only artery to the liver[1-3]. This is found by palpating for a pulse behind the portal vein. If the arterial anatomy is normal, the gastroduodenal artery is then ligated and divided, there by bringing into view the portal vein. The oesophagus is taped with a red rubber for retraction to facilitate the dissection of the supracoeliac aorta, which is mobilized by incising the right diaphragmatic crus between the oesophagus and the inferior vena cava. The SMA is dissected to its origin from the aorta. It is essential to examine again for an abnormal left hepatic artery arising from left gastric artery[2] before dissecting the tissues around the oesophagus. A tape is then passed around infradiaphragmatic aorta. 3 bowls with ice slush are prepared on the bench table, two containing one sponge and the other containing four sponges. If cardiac transplantation is also occurring, the heart teams come in at this stage and prepare their organ for retrieval.
The donor is systemically heparinised at a dose of 300 U/kg body weight, lasting about 3 min. A large cannula (Wire Reinforced Venous Return Catheter with TF 28 diameter and 35 cm in length) previously flushed with preservation solution is placed in the distal aorta and fixed; care should be taken not to insert the tip of the cannul a beyond the origins of the renal arteries. The lower end of the IVC is tied off with the silk tie in place, and a large aortic clamp is simultaneously used above supraceliac artery to cross clamp. The aorta line clamp is then opened to flush the liver with 4 liters Ross solution. The suprahepatic inferior vena cava is opened at its entrance to the heart and blood is vented into the pericardium and pleural space. Cold packs are put over and under the liver and also over the kidneys and slush are poured into the abdominal cavity to facilitate rapid cooling of the viscera. Four litres of Ross solution and two litres of UW solution (each liter of UW solution containing 15 mg of dexamethasone and 40 units of insulin) are used for the perfusion.
When the effluent clears and the liver is cold, in the bloodless field, the intrahepatic IVC is opened and transected above the renal veins. The diaphragm is divided on the left side down to the aorta avoiding the oesophagus. The pericardium and the posterior wall of the suprahepatic IVC are divided. The hepatic hilar dissection commences with dissection of the common bile duct (CBD) inferiorly. It is divided low behind the head of the pancreas, the stump being marked with a 5-0-prolene stitch. The SMV is followed behind the head of pancreas towards the portal vein. The branches of SMV are dissected and ligated. The lower part of SMV is divided, and the origin of spleen vein is ligated and dissected. The PV and SMV are harvested together. The aorta is opened from the bifurcation to the origin of SMA. The LGA and the splenic art ery are ligated and divided close to the stomach and the splenic hilum, respectively. The possible existence of an abnormal right hepatic artery from the SMA still needs to be ruled out by careful examination. The duodenum is then dissected off the pancreas. The origin of the coeliac artery is identified within the opened aorta and cannulated with an infant feeding tube probe to ensure the supply to the liver. The presence of any accessory hepatic arteries from either the SMA or the aorta must be excluded again[3]. If no abnormal arterial anatomy is found, a patch of aorta and SMA combining the coeliac artery and the common hepatic artery with a part of pancre as are completely retained until the next step that is to remove en bloc. The right diaphragm is incised from anterior to posterior down towards the upper pole of the right kidney, age nerous patch of diaphragm left attached to the liver. The right side is protected by gentle down ward traction, with careful division of the hepatorenal ligament. When the diaphragmatic attachment of the liver freed, the liver vasculature is delineated; the remaining diaphragamatic and peritoneal attachments are divided. The liver is removed and put into an ice bowel over a cold abdominal pack containing cold normal saline solution. The portal vein is cannulated and perfused with some UW solution, and the liver is put into a bag and packed for transport in this UW solution. Both iliac arteries and veins are routinely removed from the donor for possible use in artery or portal vein reconstruction in the recipient. These vessels can be preserved in specimen bottles containing UW solution and transported in the same ice chest as the liver.
Trimming of the liver is carried out under in a sterile operating theatre, the liver being kept in the same basin containing cold UW solution. The aorta is first divided and infant-feeding tube 5F is used to delineate arterial anatomy from the aortic patch towards the liver. The tissue around the vessels is excised, and all of the major arterial branches are ligated except for one or two, which may be left to check after the anastomosis. The divided common bile duct is flushed with cold saline using the larger infant feeding tube until the effluent clear. The biliary tract is not usually dissected to prevent devascularisation of the bile duct. Further preparation of the common bile duct is left until biliary reconstruction is being performed in the recipient. The inferior vena cava is prepared by excising the patch of diaphragm around the suprahepatic vein lumen. Excising the adrenal tissue and carefully ligating the right adrenal veins similarly cleans the infrahepatic ven a cava. Sometimes it is helpful for the operator to insert their finger into the lumen of inferior vena cava to provide guidance to the dissection. All of the redundant tissues attaching the liver should be cleaned up, and the opening of the vessels should be sutured with 5-0 prolene sutures. Liver biopsy is routinely done. The Donor liver is also weighed and recorded. The first bag containing the donor liver is tied and put into another bag containing cold saline and the 2nd bag is tied again and put into the 3rd bag and tied. The bags are placed in a box containing ice with the lid tightly sealed and the bag placed into a large box containing ice.
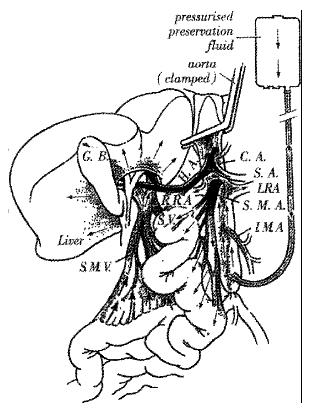
Since the earliest description of a standardized technique for multiple organ procured by Starzl et al[4], modifications have been suggested to simplify the operative methods and minimize the risk of damage to the graft[5-7]. Ville de Goyet et al[8] have previously reported that APO has no detrimental affect on the graft liver function in either adult or pediatric transplant patients. Effective liver perfusion occurs in the APO via the aorta and hepatic artery, but also via the portal vein after the fluid has traversed the intestinal circulatory bed (Figure 1). The donor liver is in fact perfused with the cold solution from both of HA and PV, thereby rendering the liver with APO safe for grafting as shown in our previous report[9].
The advantage of the technique of aortic perfusion only is the avoidance of the need to undertake the additional dissection and vessel cannulation required for a technique involving portal vein perfusion. The method has simplified the operative procedure, as it involves flushing through an aortic cannula only, instead of having the two cannulae and perfusion sets needed for the portal vein perfusion technique. In the critically unstable donors these methods have been shown to be beneficial.
The APO method can minimize the risk of damage to the graft. The conventional portal vein perfusion technique requires dissection especially around the SMA prior to its ligation. Such dissection may result in a higher incidence of hepatic arterial injury[1-3,10]. The risk of surgical error is especially significant when the right hepatic artery arises from the SMA. This vascular anomaly occurs in about 9%-15% of the population[1,6]. With regard to the other abdominal organs retrieved during multiorgan procurement, the vascular anatomy can be defined on a side table allowing the best setting for the most appropriate division of common vascular supplies, which reduce the danger of injured vessels as well. Another advantage of the APO method is to decrease the risk of manipulative arterial spasm with resulting is chaemic damage to the organs.
The method also allows the other organs of donor to be utilized. The procedure of perfusing the liver allows the other abdominal viscera, including the pancreas, intestine and kidneys, to be flushed with a perfusion solution at the same time. The procurement of abdominal multiorgan en bloc combined liver; pancreas, kidneys and intestine can be performed in a single donor.
The presence of a normal blood supply is very important for a liver following transplantation. Any procedure resulting in damage to the arterial supply should be avoided at all costs. Special attention should be paid to the possible presence of accessory hepatic arteries from the SMA, LGA and aorta. During prolonged back-table procedures involving arterial reconstructions, hypothermia should be also strictly maintained(Table 1).
| CA | - | celiac artery | SA | - | splenic artery |
| HA | - | common hepatic artery | SMA | - | superior mesenteric artery |
| SMV | - | superior mesenteric vein | IMA | - | inferior mesenteric artery |
| LRA | - | left renal artery | RRA | - | right renal artery |
| SV | - | splenic vein | GB | - | gall bladder |
Edited by Wang YQ
| 1. | Miller C, Mazzaferro V, Mackowka L. Rapid Flush Technique for Donor Hepatectomy: Safety and Efficacy of an Improved Method of Liver Recovery for Transplantation. Transplant Proc. 1988;20:948-950. [PubMed] |
| 2. | Todo S, Makowka L, Tzakis AG, Marsh JW, Karrer FM, Armany M, Miller C, Tallent MB, Esquivel CO, Gordon RD. Hepatic artery in liver transplantation. Transplant Proc. 1987;19:2406-2411. [PubMed] |
| 3. | Ferla G, Colledan M, Doglia M, Fassati LR, Gridelli B, Rossi G, Caccamo L, Gatti S, Galmarini D. Back-table surgery for liver grafts. Transplant Proc. 1988;20:1003-1004. [PubMed] |
| 4. | Starzl TE, Hakala TR, Shaw BW, Hardesty RL, Rosenthal TJ, Griffith BP, Iwatsuki S, Bahnson HT. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223-230. [PubMed] |
| 5. | Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343-348. [PubMed] |
| 6. | Sheil AG, Thompson JF, Stephen MS, Graham JC, Eyers AA, Bookallil M, Kalpokas M, McCaughan GW, Dorney SF, Ekberg HB. Liver graft revascularization by donor portal vein arterialization following "no touch" donor hepatectomy. HPB Surg. 1988;1:57-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Lerut J, Reding R, de Ville de Goyet J, Baranski A, Barker A, Otte JB. Technical problems in shipped hepatic allografts: the UCL experience. Transpl Int. 1994;7:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | de Ville de Goyet J, Hausleithner V, Malaise J, Reding R, Lerut J, Jamart J, Barker A, Otte JB. Liver procurement without in situ portal perfusion. A safe procedure for more flexible multiple organ harvesting. Transplantation. 1994;57:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Chui AK, Thompson JF, Lam D, Koutalistras N, Wang L, Verran DJ, Sheil AG. Cadaveric liver procurement using aortic perfusion only. Aust N Z J Surg. 1998;68:275-277. [PubMed] |
| 10. | Chevallier JM, Hannoun L. Anatomic bases for liver transplantation. Surg Radiol Anat. 1991;13:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |