INTRODUCTION
Up to now, pancreatic adenocarcinoma (PAC) is still one of the leading causes of cancer death in the world, although meat progress has been made in the treatment of this disease[1,2]. Most patients with PAC are in its advanced stages and surgically unresectable at the time of diagnosis, and for those who are resected, the risk of recurrence is very high[3,4]. Consequently chemotherapy still is an alternative strategy for patients with non-resectable PAC[5-8]. However, the response to most forms of chemotherapy achieved so far is generally quite limited, and is related in part to the resistance to these chemical agents[1,5,9]. To date, some specific mechanisms of drug resistance have been elucidated, among which the best understood are increased expressions of mdr1-encoded p-glycoprotein (P-gp)[10-14], multidrug resistance protein (MRP)[15-17] and lung resistance protein (LRP)[18-20]. These membrane transporter proteins play important roles in multiple drug resistance (MDR) involving increased drug efflux and intracellular drug entrapment and/or redistribution.
Taxotere (TXT) is a member of the family of taxanes and is more potent than paclitaxel with regard to the promotion of the polymerization of tubulin, inhibition of depolymerization, and it inhibits cell replication and has greater antitumor activity in many in vitro and in vivo tumor model systems [21-24]. The drug has displayed significant antitumor efficacy against breast, lung and ovarian cancers in clinical trials[25-28], but some clinical studies do not support the use of Taxane in advanced PAC[29,30]. The studies on mechanisms by which cells acquire resistance to Taxane demonstrated that the drug is a substrate for the P170 multidrug resistance pump that is able to confer resistance to a wide variety of naturally derived hydrophobic substances[16,29,31]. Therefore, the cells selected with Taxane were found to exhibit cross-resistance to a variety of other hydrophobic drugs and have elevated levels of P-gp[32].Other possible mechanisms of resistance to TXT the include the alteration in microtubulin composition and/or dynamics[33,34] increased protein kinase C-α and -γ express ion[35] and overexpression of Bc1-2[36], and SP-gp[37]. But the exact mechanism of TXT-resistance in PAC is still not clear[32,38]. The specific mechanisms in different tumors might be different.
The mechanisms of refractoriness to chemotherapy of PAC are not fully understood. Published reports provide conflicting information regarding the expression of this MDR phonotype in human PAC[16,39]. Whether the intrinsic and acquired TXT-resistance share the same MDR mechanism is unclear, which might be important for designing a chemotherapeutic regiment and investigating appropriate reversal agents. This study was designed to investigate the mechanisms of intrinsic and acquired resistance to TXT in PAC cell line SUIT-2 and its sublines.
MATERIALS AND METHODS
Chemical reagents
Taxotere (Rh0ne-Poulenc Rorer Pharmaceuticals Inc.) was stored as 10 mmol•L¯¹ stock solution in absolute ethanol at -20 °C. These solutions were further diluted in the medium used in the cell culture immediately before each experiment. Final dilutions of 0.5-3.5 nmol•L¯¹ TXT were used for the experiments to detect the sensitivity of SUIT-2 and its sublines. Verapamil (Ve r) was purchased from American Reagent Laboratory, Inc. and Trypsin, EDTA was purchased from Gibco. MTT (3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide), rhodamine (Rho) 123 (2-[6-Amino-3-imino-3h-xanthen-9-yl] benzoic acid methyl ester), indomethacin (IMC) (1-[P-chlorobenzoyl]-5-methoxy-2-methylindole-3-acetic acid), McCoy’s 5A Medium [modified], DMEM (phenol free) medium, Fetal calf serum (FCS), sodium pyruvate, MEM amino acids (50X), MEM amino acids (100X), MEM Vitamins, L-glutamine (200 mmol•L¯¹) penicillin-streptomycin, serine, a sparagine sodium bicarbonate were purchased from Sigma.
Cell cultures
Human pancreatic cancer cell line SUIT-2 was established and supplied by Iwamura. Its sublines, including S2-007, S2-013, S2-020, S2-028, were cloned by soft agar culture and shown to have different metastatic potential[40]. These cell lines were cultured and the TXT resistant SUIT-2 cell line (S2/TXT) was developed as described previously[41].
MTT colorimetric assay
The MTT colorimetric assay was performed as described previously[41]. Briefly, when the cells of SUIT-2 and its sublines reached 85%-90% confluency, they were detached from the flasks with 0.25% trypsin and resuspended in the culture medium. Cells were grown within 9 6-well microtitre plates (Costar) in 2 × 1010 cells•L¯¹ of culture medium each well and acclimated for 6 h, and 100 μL of various concentrations of drugs diluted in culture medium was added. To study the effect of Ver and IMC on TXT cytotoxicity, 1 μ mol•L¯¹ Ver or 10 μmol•L¯¹ IMC was added with this drug. Five duplicate wells were used for each determination. The plates were incubated for 72 h when the control cells reached 90% confluency, and 30 μL of MTT in PBS solution was then added to each well and the plates were incubated for another 4 h. The medium and MTT solution were then aspirated and 150 μL of dimethyl sulfoxide (DMSO) (Sigma) was added. The plates were read on Bio-Tek Microplate reader EL 800 (Bio-Tek Instruments, Inc). Fraction of cell proliferation was defined as the ratio of optical density volume to that of controls. The IC50 was defined as the concentration of the drugs required to reduce the absorbance by 50% in treated cells as compared to that of the controls.
Rho-123 accumulation assay
Rho-123 accumulation was determined by flow cytometry. Briefly, SUIT-2, S-020 and S2/TXT cells in logarithmic growth phase were harvested with trypsin and resuspended in phenol red-free DMEM medium at 1 × 109 cells•L¯¹. Aliquots of 1 mL cell suspension were preincubated with or without Verapamil for 45 min and then 200 μg•L¯¹ Rho-123 dissolved in DMEM was added and incubated for 40 min. After incubation, cells were washed and resuspended in ice-cold Rho-free DMEM with 5 μmol•L¯¹ Ver. The accumulation of Rho-123 in cells was analyzed with flow cytometry. Ten thousand cells per sample were analyzed. Cells of these cell lines, which had not been ex posed to Rho-123, were used to determine the background of autofluorescence.
RT-PCR
The isolation of total RNA was based on the method of Chomczynski et al[40]. Equal amounts of RNA were reversed transcribed using SuperScriptTM One-stepTM RT-PCR System (Life Technologies). Twenty-five μL PCR mixed in each tube containing: 0.5 μL RT/Tag Mix, 3 μL of 5 mmol•L¯¹ MgSO4, 5 μ L diethy pyrocarbonate (DEPC, Sigma)-treated distilled water, 3 μL mixed primer pairs, 12.5 μL 2X Reaction Mix and 1 μg Template RNA in DEPC water. After an initial denaturation in a programmable thermocycler at 94 °C for 2 min, PCR was carried out for 30 cycles with the thermal profile: denaturing at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min with an extra-10 min extension for the last cycle. After completion of the amplification cycles, 5 μ L of each PCR product was electrophoresed at 60 V for 1.5 h on a 1.2% agarose gel (GIBCOBRL) in Trizma base and Glacial acetic acid EDTA buffer. Both target and control (β-actin) gene sequences were coamplified in the same tube. Gene expression was normalized to β-actin transcript; this was noted for REL for relative expression levelg (REL = densitometric value of studied gene/densitometric value of β-actin). The specific primers for mdr1, MRP and LRP used in this study were as described as before[41]. The beta-tubulin isotype sequences were designed as follows: The sense primer sequence was 5’-CAACAGCACGGCCATCCAGG-3’. The antisense primer sequences were M40 (Class I): 5’-AAGGGGCAGTTGAGTAAGACGG-3’, β9 (Class II): 5’-GTAGAAAGACCATGCTTGGG-3’, β4 (Class III): 5’-CTTGGGGCCCTGGGCCTCCGA-3’, 5β (C lass IVa): 5’-AAGTAGCCAGAGGTAAAGCGAG-3’, β2 (Class IVb): 5’-CTTTCCCCAGTGACTGAAGG-3’. β-actin was used as control. Its sense primer was: 5’-TGACGGGGTCACCCACACTGTGCCCATCTA-3’; and antisense primer was: 5’-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3’.
RESULTS
Sensitivity of SUIT-2 and its sublines to TXT
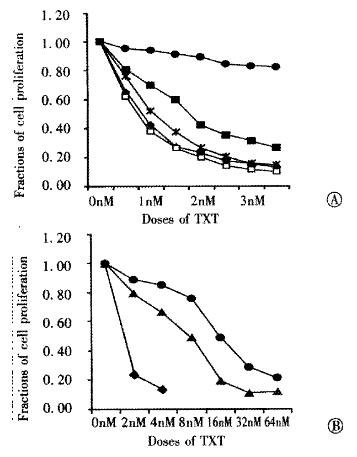
To identify for intrinsic TXT-resistant PAC cell lines, SUIT-2 and its sublines (S2-007, S2-013, S2-0 20 and S2-028)) were investigated as to their sensitivity to TXT as by MTT assay. The innate sensitivity of SUIT-2 and its sublines to TXT were found. S2-020 cells were most resistante to TXT compared with its parental cell line SUIT-2 (IC50 0.85 nmol•L¯¹), and other cell lines. IC50 of S2-020 (16.2 nmol •L¯¹) was 19-fold to that of SUIT-2. The second most resistant cell line was found to be S2-007 (IC50: 1.82 nmol•L¯¹). The other cell lines had no significant resistance to TXT. The IC50 of S2-013 and S2-028 were 0.75 and 1.2 nmol•L¯¹, respectively. IC50 of the acquired TXT resistant cell line established from SUIT-2 was 8.1 nmol•L¯¹, 9.5- fold to its parental cell line SUIT-2 (Figure 1).
Figure 1 Dose-response curves of SUIT-2 and its sublines for taxotere.
◆: SUIT-2, ■: S2-007, : S2-013, ●: S2-020, : S2-028, △: S2/TXT.
Reversal effects of Ver and IMC on the resistance to TXT
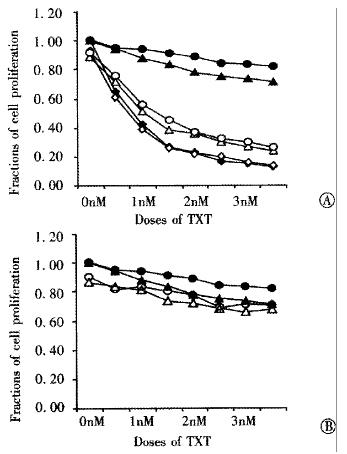
In order to elucidate the function of drug transporter pump P-gp and MRP expressed in the cell line S2-020 and S2/TXT, their corresponding blockers Ver and IMC were used respectively. Figure 2A shows that Ver at a concentration of 1 μmol•L¯¹ can almost completely reverse the resistance of S2-020 and S 2/T XT to TXT, but the same concentration of Ver had no sensitizing effect to SUIT- 2, which did not express P-gp. IMC, a specific modulator of MRP, had no reversal effect on the TXT-resistance found in these cell lines, although they all expressed MRP (Figure 2B).
Figure 2 Reversal effects of Verapamil and Indomethacin on TXT -resistance.
S2/TXT (▲), and S2-020 cell lines (●) were compared to SUIT- 2 (◆). Ver (Figure 2A) and IMC (Figure 2B) have been shown different sensitizing effects of TXT-resistance in S2/TXT and S2-020 (open symbols).
Rho 123 accumulation and efflux
Accumulation and efflux of Rho-123, which is related to the transporter activity of P-gp, from SUIT-2, S2/TXT and S-020 cells as tested by flow cytometry. The accumulation of Rho-12 3 in SUIT-2 cells is much higher than that of S-020 and S2/TXT cells. The addition of 5 μmol•L¯¹ Ver led to significantly increased intracellular Rho 123 levels in the TXT-resistant S2-020 and S2/TXT cells but not in the TXT-sensitive SUIT-2 cells and its other cell sublines.
Expressions of mdr1, MRP, and LRP
The expressions of three major drug transporter pump genes mdr1, MRP, L RP were studied by RT-PCR. Figure 3 shows that there is a strong expression of mdr1 in both TXT-resistant cell line S2-020 and S2/TXT, no expressions in their parental cell line SUIT-2 and other two subline S2-013 and S2-028, which are most sensitive to TXT. MRP and LRP expressed in most of these cell lines, but no relationship was found between their expression and TXT-resistance.
Figure 3 Expressions of mdr1, MRP, LRP in SUIT-2 and its sublines by RT-PCR analysis.
Expressions of β-tubulin isotypes
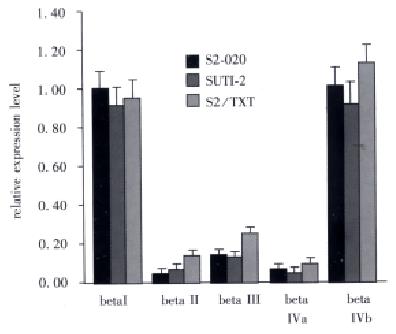
β-tubulin isotype transcript analysis was performed by using isotype-specific primers and the RNA from SUIT-2 and its two TXT-resist ant cell line S2-020 and S2/TXT. Densitometric analysis of expression levels of each isotype was quantitated relative to the expression of the control gene β actin by calculating the ratio of the target gene to the control gene PCR product. As shown in Figure 4, the βI and βI Vb were the predominant transcript in all the three cell lines. A 2.4-fold increase of β II and a 2.3-fold increase of β III compared to SUIT-2 cell line were seen in S2/TXT cell line.
Figure 4 The RT-PCR analysis of the β tubulin isotype transcripts for the SUIT-2 and its two TXT- resistant cell lines.
The relative expression levels of transcripts were expressed as a ratio of denitomet ric value of studied gene to that of β actin.
DISCUSSION
An increasing use of Taxanes in the treatment of variety of cancer including advanced PAC, has highlighted the need to elucidate the mechanism responsible for the development of resistance to this kind of drugs. Multidrug resistance may develop through a variety of mechanisms, which may include the alterations of extracellular drug efflux, intracellular extrapment or redistribution, drug detoxification, nuclear target and apoptotic response[42]. With respect to taxane-related resistance, many studies have shown that it is often associated with the expression of mdr1[29,32], but the expression of the mdr1 gene only accounts for part of the resistance mechanisms to taxanes. In a study by Dumontet et al[33], only 44% of resistant clones were found to express the mdr1 gene, and accumulation studies with labeled PTX did not show altered accumulation in mdr1 negative clones. Such observations indicate that there must be other mechanisms involved in taxanes resistance. Whether or not other drug transporters including MRP and LRP are also involved in this mechanism is not clear. There are reports that cell lines with MRP or LRP phenotype also confer a low level of resistance to taxane[43], though most findings showed that taxane-resistance is independent of MRP expression [44,45].
In this study, both intrinsic and acquired TXT resistant cell lines, which were derived from the same parental cell line, have expression of mdr1 gene, and also these resistances can be reversed almost completely by a P-gp blocker Verapamil. Active drug transporter pump in these TXT-resistant cells was also shown by Rho-123 accumulation assay. Though most of cell lines expressed MRP and LRP as shown by RT-PCR, they had no relationship with the TXT-resistance in these cell lines. To confirm that no MRP mediated TXT resistance exists in these cell lines, we used Indomethacin, a specific inhibitor of MRP[46] and found no sensitizing effect to TXT cytotoxity. LRP positive cell line SUIT-2 had the same sensitivity as LRP negative S2-028 cell lines, showing that there is no active LRP mediated TXT-resistance. These data indicated that the TXT-related drug resistance in these cell lines is mainly mediated by P-gp. The high incidence of MRP and LRP expressions in these PAC cell lines might be involved in other kinds of intrinsic drugs resistance. Recently, other studies on the mechanisms involved in taxane-resistance have been focused on the composition and the mutations in β-tubulin isotypes[32,33,47-49]. The possible mechanisms involved in the induction of resistance to taxanes may include altered metabolism and/or subcellular distribution, altered interaction between the tubulin-binding agents and microtubules, and altered response to cell cycle arrest by mitotic blockage.
The taxanes are unique among tubulin-targeted cytotoxins so far as they bind to polymerized tubulin only[24,50]. Direct photoaffinity labeling has demonstrated that taxane binds preferentially to the β subunit of the microtubule[51]. Sequence analysis identified the photoaffinity labeled amino acid residues β270 and β364 as important modulators of paclitaxel’s interaction with the tubulin molecule[47]. The direct proofs that altering the isotype composition of a cell affects its sensitivity to any antimitotic drug have been reported, but the results were conflicting[32,33,47,48,52]. Virtually all major β-tubulin isotypes have been reported to be changed in various drug resistant mutants[32-34,47-49,52], and this runs counter to the idea that specific isotype of β-tubulin confers altered sensitivity to these drugs. Currently, more direct evidences that altered expressions of β-tubulin isotype in taxane-resistance cells contribute to their resistance phenotype have been demonstrated. Blade et al[32] by transfecting Chinese hamster ovary cells with cDNA encoding epitopetagged class I, II, and IV bβ tubulins found that the production of βI, βII, or βIVb tubulin had no effect on the sensitivity of the cells to PTX. However, Kavallaris et al[53] designed antisense phosphorothioate oligode oxynucleotides targeted against resistant lung cancer cells, and demonstrated that a decrease in class IIIβ tubulin mRNA and protein expression which corresponded to a 39% decrease sensitivity to PTX.
Different mechanism of taxane-related drug resistance may be tumor (or cell line)-dependent or even cell-dependent. Tumor histologic origination and heterogeneity of tumor cells may be responsible for this difference[33]. Since P-gp was often expressed in normal pancreatic tissue[54], P-gp mediated TXT resistance can be found natively in PAC or be readily induced by their substrates. This study showed the changes of β-tubulin isotype not only in the P-gp-negative taxanes-resistance cells as in other studies[47,48], but also in P-gp-positive cells. However, the S2-020/TXT cells with both P-gp expression and changes of tubulin isotype profile did not show an additive or synergetic effects on TXT-resistance resistant to TXT compared with only P-gp-positive cell line S2-020. On the other hand, this resistance can be fully reversed by P-gp reversal agent Verapamil, suggesting that the change of β-tubulin isotypes might be a collateral change and not necessarily responsible for TXT-resistance in this cell line. Since many critical cellular functions, such as cell movement, mitosis, and maintenance of cell structure are associated with cytoskeletal elements in which the microtubular system plays a major role, it is not clear whether the changes of tubulin in this TXT selected cell line are involved in other biologic behavior of cells, such as cell mobility and invasiveness[55-58], which need to be further investigated.
The different expression of P-gp or tubulin mutation may also be related to the means by which the resistant cells were selected. Multiple step selected cells often present high levels of taxane-resistance mediated by P-gp, while single -step selection yields low level taxane-resistance cells with tubulin mutations[59], since severe tubulin mutations are very likely to affect cell survival and will be lost during the selection. The cells with different mechanisms of taxane-resistance may have different characteristic. TXT-related drug resistance mediated by P-gp often presents a cross-resistance to other natural chemical agents, which is different from the taxane-resistance induced by the changes of β-tubulin isotype profile, the latter was often only cross-resistant to other kinds of anti-microtubule agents, such as Vinca alkaloids[33]. However, additive and synergetic effects between Vinorelbine and TXT on human lung cancer[60] seems that their combinations are not contraindicated. Another study has shown that resistance to taxanes can be reduced by increasing the duration of exposure in P-gp expressing cells, but not in the taxane-resistance cell line which does not express P-gp[61]. Since the resistance was highly drug specific and none of the cell lines was resistant to all drugs, identifying specific mechanism of drug resistance phenotype in certain tumor is helpful in selecting non-cross-resistant regiments and appropriate reversal agents.