Published online Dec 15, 2001. doi: 10.3748/wjg.v7.i6.741
Revised: November 19, 2001
Accepted: November 25, 2001
Published online: December 15, 2001
There are two common types of adult patient with a short bowel, those with jejunum in continuity with a functioning colon and those with a jejunostomy. Both groups have potential problems of undernutrition, but this is a greater problem in those without a colon, as they do not derive energy from anaerobic bacterial fermentation of carbohydrate to short chain fatty acids in the colon. Patients with a jejunostomy have major problems of dehydration, sodium and magnesium depletion all due to a large volume of stomal output. Both types of patient have lost at least 60 cm of terminal ileum and so will become deficient of vitamin B12. Both groups have a high prevalence of gallstones (45%) resulting from periods of biliary stasis. Patients with a retained colon have a 25% chance of developing calcium oxalate renal stones and they may have problems with D (-) lactic acidosis. The survival of patients with a short bowel, even if they need long-term parenteral nutrition, is good.
- Citation: Nightingale JMD. Management of patients with a short bowel. World J Gastroenterol 2001; 7(6): 741-751
- URL: https://www.wjgnet.com/1007-9327/full/v7/i6/741.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i6.741
A patient has a short bowel when there is an insufficient length of functioning gut to allow adequate absorption, somacronutrient and/or water and electrolyte supplements are needed to maintain health and/or growth. If untreated or without compensatory mechanisms undernutrition and/or dehydration result[1]. This is likely to be the case if less than 200 cm small bowel remains. The problems experienced by patients with a short bowel depend upon the type and length of remaining small bowel and the presence or absence of a functioning colon. Patients traditionally described as having the short bowel syndrome were those with a retained colon who had intractable diarrhoea with malabsorption of fat, vitamin, and other nutrients so leading to undernutrition, with continuing weight loss and anaemia[2]. The normal adult human small intestinal length varies from about 275-850 cm as measured from the duodeno-jejunal flexure at autopsy or surgery and tends to be shorter in women[3-6]. Congenital cases of a short bowel have been reported and are usually associated with malrotation of the gut[7]. Patients who start with a small intestinal length at or below the lower end of the normal range may develop the problems associated with having a short bowel after relatively little small intestine has been removed[8]. It is important to refer to the remaining ength of small intestine rather than to the amount resected.
Ileal mucosa, in contrast to the jejunal mucosa, has “tight” intercellular junctions and thus can concentrate its contents. However, jejunal mucosa has “leaky” intercellular junctions and so the osmolality of the luminal contents is similar to that of plasma. Gastrointestinal transit is naturally slower in the ileum than jejunum so allowing more time for absorption[9,10]. The terminal ileum has the specific functions of absorbing vitamin B12[11,12] and bile salts. Any ileum remaining, after a small bowel resection, can structurally and functionally adapt so increasing absorption[13-16], while remaining jejunum can only functionally adapt if some ileum or colon also remains[16-19]. Thus the outcome is more favourable after a jejunal than an ileal resection.
Preservation of the ileocaecal valve has traditionally been thought to slow transit and prevent the reflux of colonic contents into the small bowel. However, resection of the ileocaecal valve in adults does not affect these functions[9,20].
Conservation of the colon is advantageous as it absorbs water, sodium[21-25], magnesium[25,26], calcium[27], short and medium chain fatty acids[28-30], slows gastro-intestinal transit[31] and stimulates small intestinal hyperplasia[32]. Patients with a preserved colon after a small bowel resection may survive without parenteral support with a very short[33,34] or even no remaining jejunum[35].
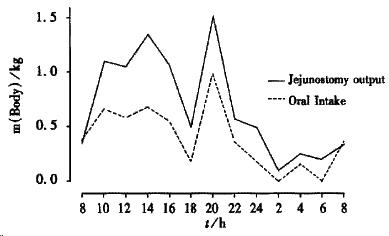
There are three types of patient with a short bowel. Patients in whom the ileum, often with the ileocaecal valve has been removed leaving a jejuno-colic anastomosis (jejunum-colon); patients in whom some jejunum, the ileum and colon have been removed, so they have an end jejunostomy and patients who have had a predominantly jejunal resection, and have more than 10 cm of terminal ileum and the colon remaining (jejuno-ileal)[25,36,37]. Jejuno-ileal anastomosis patients are not common and as their management is broadly similar to those with a jejuno-colic anastomosis, they are not specifically discussed. Patients with a jejunostomy can be classified as net “absorbers” or net “secretors”. The net “absorbers” absorb more water and sodium from their diet than they take orally (usual stomal output about 2 kg/24), thus they can be managed with oral sodium and water supplements and parenteral fluids are not needed. They usually have more than 100 cm residual jejunum. The net “secretors” usually have less than 100 cm residual jejunum and lose more water and sodium from their stoma than they take by mouth (usual stomal output may be 4-8 kg/24). The jejunostomy output from a net “secretor” increases markedly in the daytime in response to food and is minimal at night[38] (Figure 1). The three most common reasons for patients having less than 200 cm of small bowel are superior mesenteric artery thrombosis, Crohn’s disease and irradiation damage[25,36,37,39] (Table 1). A short bowel more commonly arises in women (67%) thao men[25], this may be because women start with a shorter length of small intestine than men.
| Jejunum-colon | Jejunostomy | |
| Total (sex) | 38 (26F)* | 46 (31F) |
| Age | 46 (7-70) | 42 (16-68) |
| Median jejunal length (cm) | 90 (0-190) | 115 (20-190) |
| Diagnosis | ||
| Crohn’s disease | 16 | 33 |
| Ischaemia | 6 | 2 |
| Irradiation | 5 | 3 |
| Ulcerative colitis | - | 5 |
| Volvulus | 5 | - |
| Adhesions | 4 | 1 |
| Diverticular disease | 1 | 1 |
| Desmoid tumour | 1 | 1 |
The problems that arise after a major intestinal resection reflect both normal and altered physiology. Most experimental work in animals involves a predominantly jejunal resection (jejuno-ileal anastomosis), which is not the common situation in man.
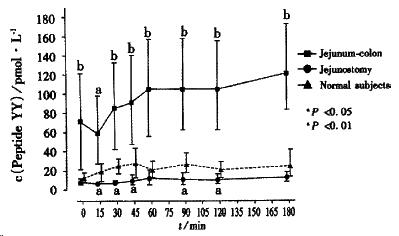
Gastric emptying and small bowel transit of liquid is fast in patients with a jejunostomy and normal in patients with a retained colon, probably due to circulating plasma levels of peptide YY being low and high respectively[40] (Figure 2). Peptide YY is an enteric hormone produced by the L cells in the terminal ileum and colon upon exposure to luminal nutrients, it slows gastric emptying and gastrointestinal transit[41].
Salivary secretion is reduced in patients with a jejunostomy[42]. Gastric acid secretion is increased in dogs with denervated gastric pouches. In man some cases of long-term survival in patients with a short bowel has been attributed to their previous gastric surgery[43-45]. High circulating gastrin levels are observed[40,46,47] and may be due to less small bowel being available to catabolize gastrin[48,49]. Gastric acid hypersecretion could increase the incidence of peptic ulceration[50]; impair nutrient absorption by causing bile salt precipitation[51]; reduced pancreatic enzyme function and increased jejunal motility. However although there may be an increase in gastric acid secretion in the first 2 wk after a small bowel resection[52], there is no good evidence for gastric acid hypersecretion in man in the long-term, especially in those with a jejunostomy[53]. Pancreatic function is reduced by undernutrition[54] and if there is no oral intake[55], but not after a small bowel resection leaving the colon[56].
The ileum absorbs vitamin B12 and bile salts. If more than 60 cm of terminal ileum is resected vitamin B12 deficiency is likely to occur[11,12,57]. Fat is absorbed over a longer length of the intestine than carbohydrate or protein and this distance increases as oral intake rises[58]. When more than 100 cm terminal ileum has been resected the increased hepatic synthesis of bile salts is not enough to keep up with the stool or stomal losses and a degree of fat malabsorption results[59].
Intestinal adaptation is the process that attempts to restore the total gut absorption of macronutrients, macrominerals and water, to that of before the intestinal resection[60]. This may occur by the patient eating more food than normal (hyperphagia)[61]. The remaining bowel may increase its absorptive area (structural adaptation) and/or gastrointestinal transit may slow (functional adaptation). After a jejunal resection in animals the ileal remnant undergoes structural changes, which include elongation of villi, deepening of the crypts and an increase in the number of enterocytes along a given length of villus. This structural adaptation may relate to circulating plasma levels of glucagon-like peptide-2 (GLP-2). GLP-2 is an enterocyte specific peptide growth hormone produced by the L cells in the terminal ileum and colon and it causes small and large bowel villus and crypt growth[62].
Jejunum-colon In patients with a jejuno-colic anastomosis, no structural adaptation has been demonstrated[17,63,64] even though high GLP-2 levels are observed[65]. Functional adaptation with slowing of gastric emptying and small bowel transit may occur[31] and may be due to high circulating peptide YY levels[40]. In these patients there is a small reduction in faecal weight in the 3 mo following the small bowel resection[16]. There is increased jejunal absorption of macronutrients (e.g. glucose), water, sodium and calcium with time[15,18,19,66], and an increased chance of the patient being able to stop parenteral nutrition[25,36,37,67]. The intestinal calcium absorption may continue to increase for more than two years after a resection[66].
Jejunostomy Although adaptation occurs in the months after the creation of an ileostomy, there is no evidence for any structural[53] or functional[25,68] adaptation at any time in patients with a jejunostomy. This may be because patients with a jejunostomy have low circulating plasma levels of GLP-2 and peptide YY[40,69,70].
Jejunum-colon patients often appear well after their resection except for diarrhoea/steatorrhoea, but in the following months may lose weight and become severely undernourished. Patients with a jejunostomy have problems of dehydration immediately after surgery due to large stomal water and sodium losses. This jejunal output is greatest after food and drink have been consumed. The clinical assessment of a patient with a short bowel includes a measurement of residual small bowel length and an assessment of water, sodium, magnesium and nutritional status. The state of hydration and magnesium balance is of immediate importance to those with a jejunostomy.
From knowledge of residual bowel length predictions about the long-term need for fluid/nutrition can be made (Table 2).
| Jejunal length (cm) | Jejunum-colon | Jejunostomy |
| 0-50 | PN | PN+PS |
| 51-100 | ON | PN+PS* |
| 101-150 | None | ON+OGS |
| 151-200 | None | OGS |
Anatomical length The remaining small bowel length can be measured at surgery (10-30 cm segments are measured along the anti-mesenteric border with great care being taken not to over-stretch the bowel). If not measured at surgery, an opisometer, a device used for measuring distances on maps can be used to trace the long axis of the small bowel on a small bowel meal radiograph. This technique is relatively accurate if the total small intestinal length is less than 200 cm and if the entire small bowel is shown on one film[36,71].
Functional length Citrulline is a non-essential amino acid synthesised from glutamine and all the citrulline in the systemic circulation is derived from the enterocytes. Post absorptive plasma levels of citrulline relate to the length of remaining functional small bowel[72].
Water and sodium deficiency (most common in jejunostomy patients) may result in hypotension and pre-renal failure. Daily body weight and accurate fluid balance (including stomal output) are the most important measurements. The serum creatinine, potassium and magnesium, and urinary sodium may be measured every 1-2 d initially, then once or twice a week and if long-term at home every 2-3 mo. Water and sodium deficiencies are detected by an abrupt fall in body weight, postural systolic hypotension (1.33 kPa), low urine volume and, if severe, by a rising serum creatinine and urea. The most helpful early measure of sodium depletion is the sodium concentration in a random urine sample. A concentration of 0-5 mmol•L¯¹ suggests sodium depletion. The aims are to maintain hydration/body mass and a daily urine volume of at least 801 mL with a sodium concentration greater than 20 mmol•L¯¹.
Magnesium depletion is common especially in patients with a high stomal output. A serum value of less than 0.6 mmol•L¯¹ may give rise to symptoms.
A patient may be assessed as undernourished if the body mass index is less than 18.5 kg•m-2, if there is a recent weight loss of more than 10% or if the mid-arm muscle circumference is less than 19 cm in a woman or 22 cm in a man[73]. The degree of malabsorption and thus oral enteral nutrition requirements can be predicted from knowledge of the residual length of small bowel (Table 2). In terms of energy absorption parenteral nutrition is needed if a patient absorbs less than one-third of the oral energy intake[38,74]; though in people with high energy needs parenteral nutrition may be needed at a higher percentage energy absorption.
Jejunum-colon The colon has a large capacity to absorb sodium and water; thus patients with a short bowel and a preserved colon are rarely in negative water and sodium balance and rarely need water or sodium supplements[23-25]. Although the colon secretes potassium, a low serum potassium level rarely occurs[25]. If sodium depleted, a glucose-saline drink can be sipped during the day as for patients with a jejunostomy. There is an exchange mechanism of chloride for bicarbonate in the colon; thus if much sodium chloride is consumed, bicarbonate may be lost in the stools, giving rise to a metabolic acidosis.
Jejunostomy Patients with a jejunostomy have a large volume of stomal output, which is greater after eating or drinking. Each litre of jejunostomy fluid contains about 100 mmol•L¯¹ of sodium[38]. This high volume output is mainly due to a loss of the normal daily secretions (0.5 L saliva, 2.0 L gastric juice and 1.5 L pancreatico-biliary) produced in response to food and drink. Each day about 6 litres of chyme pass the duodeno-jejunal flexure, but by 100 cm distal to the duodeno-jejunal flexure the chyme volume is about the same as that of the oral intake[75,76]. If a jejunostomy is at a small bowel length of less than this more emerges from the stoma than is taken in by mouth (“secretors”). Even in the fasting state there is an obligatory loss of intestinal secretions produced with the migrating myoelectric complex (MMC)[77]. The effluent from a jejunostomy or ileostomy contains relatively little potassium (about 15 mmol•L¯¹)[23,24,38]. Potassium balance is not often a problem and net loss through the stoma occurs only when less than 50 cm jejunum remains[38]. A low serum potassium level is most commonly due to sodium depletion with secondary hyperaldosteronism and thus greater than normal urinary losses of potassium[23]. Hypokalaemia can also be due to hypomagnesaemia, which causes dysfunction of potassium transport systems and increases renal potassium excretion; this hypokalaemia is resistant to potassium treatment but responds to magnesium replacement[78,79]
It is helpful both in predicting outcome and in choosing the type of nutritional support if the length of bowel above the stoma/fistula is known. Contrast studies (small bowel meal, fistulogram or stoma enema) may help with this. An examination of the output (colour/consistency/24 h volume) gives an indication of the internal origin of the fistula.
A stoma may produce a high output if there is intra-abdominal sepsis, enteritis (e.g. clostridium or salmonella), partial/intermittent bowel obstruction, recurrent disease in the remaining bowel (e.g. Crohn’s disease or irradiation) or sudden stopping of drugs (steroids or opiates).
The concentration of sodium in the output remains constant (about 100 mmol•L¯¹) whatever treatment is given. While there is a small stomal loss when fasting, the greatest increase in stomal output is after food or drink (Figure 2)[38]. Even in patients with a jejunostomy and receiving parenteral nutrition, the stomal output should be reduced, as this may reduce the amount or frequency of parenteral fluid required and the social problems of managing a high output stoma. If there is marked sodium and water depletion and severe thirst, it is often necessary to establish equilibrium by giving intravenous normal saline (2-4 L•d¯¹) keeping the patient ‘nil by mouth’, which will also demonstrate that the output is driven by their oral intake. Over 2-3 d intravenous fluids are gradually withdrawn while food and restricted oral fluids are reintroduced. Great care must be taken not to administer too much fluid, which will readily cause oedema (partly due to the high circulating aldosterone levels[23,24,80,81]. To correct hypokalaemia in patients with a high output stoma, sodium/water depletion must be corrected and the serum magnesium brought into the normal range. It is uncommon for potassium supplements to be needed.
Restrict oral fluids Jejunal mucosa is ‘leaky’ and rapid sodium fluxes occur across it. If water or any solution with a sodium concentration of less than 90 mmol/L is drunk there is a net efflux of sodium from the plasma into the bowel lumen[82], until a luminal sodium concentration of about 100 mmol/L is reached. In a patient with a jejunostomy this fluid is then lost in the stomal output. It is a common mistake for patients to be encouraged to drink oral hypotonic solutions to quench their thirst, but this causes large stomal sodium losses[80,82-85]. Treatment for the high output from a jejunostomy, ileostomy or high fistula begins with the patient restricting the total amount of oral hypotonic fluid (water, tea, coffee, fruit juices, alcohol or dilute salt solutions) to less than 500 mL•d¯¹. To make up the rest of the fluid requirement the patient is encouraged to drink a glucose-saline replacement solution. Many patients at home with marginally high stoma outputs (1.0-1.5 L) will be helped by a combination of oral fluid restriction (less than 1.0 L•d¯¹) and the addition of salt to their diet. Often patients are advised to take liquids and solids at different times (no liquid for half an hour before and after food) however there is no published evidence that this reduces stomal output or increases macro or micronutrient absorption[86].
Drink oral glucose-saline solution Patients with stomal losses of less than 1200 mL•d¯¹ can usually maintain sodium balance by adding extra salt to the limit of palatability at the table and when cooking. When stoma losses are in the range 1200 - 2000 mL, or sometimes more, it is possible for a patient to maintain sodium balance by taking a glucose-saline solution or salt capsules[84]. In hot weather, due to water and sodium loss in sweat, patients with a stoma are more likely to have problems of dehydration. As the sodium content of jejunostomy (or ileostomy) effluent is relatively constant at about 90 mmol•L¯¹ and as there is coupled absorption of sodium and glucose in the jejunum[87-89]; patients are advised to sip a glucose-saline solution with a sodium concentration of at least 90 mmol•L¯¹ throughout the day. The World Health Organization (WHO) cholera solution has a sodium concentration of 90 mmol•L¯¹ [90] and is commonly used (without the potassium chloride) (Table 3). The concentration of sodium in this solution is much higher than many commercial preparations used to treat infective or traveller’s diarrhoeas. There is no evidence that the sodium bicarbonate adds to the effectiveness of this solution[89] and it may be more palatable if the sodium bicarbonate is replaced by sodium citrate. If the sodium concentration is increased further (e.g. to 136 mmol•L¯¹) absorption of sodium and water is improved[91]. However even though taste perception is different in patients who are salt and water depleted, they may find a more concentrated salt solution too salty to drink. The patient should be encouraged to sip a total of one litre or more of one of these solutions in small quantities at intervals throughout the day. As compliance is often a major problem, patients need to understand the need for the solution. To improve palatability the solution may be chilled or flavoured with fruit juice.
| Sodium bicarbonate (or citrate) | 30 mmol (2.5 gm) (2.9 gm) |
| Glucose | 110 mmol (20 gm) |
| Tap water | one litre |
| Alternative rehydration solution | |
| Sodium chloride | 120 mmol (7 gm) |
| Glucose | 44 mmol (8 gm) |
| Tap water | one litre |
A glucose-polymer (55 gm Maxijul) may be substituted for glucose to increase the energy intake by a mean of 648.8 kJ•d¯¹ (115 kcal/day)[84]. A rice powder based oral rehydration solution can further increase the amount of energy absorbed, and if there is a functioning terminal ileum one study has shown that the sodium concentration in this solution can be reduced to 60 mmol•L¯¹[92]. The glucose-polymer/rice based solution can be especially useful in diabetic patients as it causes less extreme changes in blood glucose than glucose based solutions. Sodium chloride capsules (500 mg each) are effective when taken in large amounts (14/24 h), but can cause some patients to feel sick and even vomit[84]. If an enteral feed is given, sodium chloride needs to be added to make the total sodium concentration of the feed 100 mmol•L¯¹ while keeping the osmolality near to 300 mmol•L¯¹.
Drug therapy If restricting oral fluids and giving a glucose-saline solution to drink are not adequate, drugs may be needed. As the intestinal output, especially in net “secretors” rises after meals, it is important to give the drugs before food. Drugs used to reduce jejunostomy output acttoreduce either intestinal motility or secretions. If any tablets/capsules emerge unchanged in stool/stomal output, they can be crushed, opened and/or mixed with water or put on food.
Antimotility (antidiarrhoeal) drugs Loperamide and codeine phosphate reduce intestinal motility and thus decrease water and sodium output from an ileostomy by about 20%-30%[93-95]. Loperamide is preferred to opiate drugs (e.g. codeine phosphate) as it is not sedative, addictive and does not cause fat malabsorption[94,96]. However loperamide does reduce postprandial pancreaticobiliary secretion of trypsin and bilirubin in patients with a short bowel and preserved colon[97]. They are usually taken half an hour before food. Oral loperamide 4 mg taken four times a day was more effective in reducing the weight and sodium content of ileostomy fluid than codeine phosphate 60 mg taken four times a day (94) but the effect of both together may be greater[98]. Loperamide circulates through the enterohepatic circulation, which is severely disrupted in patients with a short bowel and small bowel transit may be very rapid. Thus high doses of loperamide (e.g. 12-24 mg at a time) may be needed, as following a vagotomy and pyloroplasty[99]. These drugs are effective in most patients with a jejunostomy[38,100] particularly net “absorbers”.
Antisecretory drugs Food and drink are diluted by digestive juices, thus the volume of stomal effluent can be reduced in ‘secretors’ by drugs that reduce the secretions from the stomach, liver and pancreas. Drugs that reduce gastric acid secretion (e.g. the H2 antagonists or proton pump inhibitors or the somatostatin analogue octreotide) are most commonly used. There is one report of a patient with a jejunostomy for Crohn’s disease having gastric irradiation to successfully reduce their stomal output[101].
H2 antagonists/proton pump inhibitors Cimetidine (400 mg orally or intravenously four times a day)[102,103], ranitidine (300 mg orally twice daily)[38] and omeprazole (40 mg orally once a day or intravenously twice a day)[104,105] reduce jejunostomy output particularly in net secretors and generally in those with an output exceeding 2 litres daily. Omeprazole is readily absorbed in the duodenum and upper small bowel, but if less than 50 cm jejunum remains it may need to be given intravenously. The effect of these drugs is as good as octreotide (50 μg subcutaneously twice daily) in terms of the reduction in stomal volume[38,104]. They do not change the absorption of energy, carbohydrate, lipid, nitrogen and divalent cations[102-105] and do not reduce jejunostomy output enough to prevent the need for parenteral fluid and electrolyte replacement.
Somatostatin/octreotide Somatostatin/octreotide reduce salivary, gastric and pancreatico-biliary secretions, slow small bowel transit and may delay gastric emptying. Somatostatin has a serum half-life of 3 min so is given by continuous infusion and does reduce stomal output[106]. Octreotide has a serum half-life of 90 minutes so is given as regular (two or three time’s daily) subcutaneous injections before food. Several studies in adults have shown octreotide to reduce ileostomy diarrhoea and large volume jejunostomy outputs [100,107-114]. The greatest reductions in intestinal output have occurred in net “secretors”, and many patients have been able to reduce the volume of parenteral supplements needed[110,114]. All studies have shown a reduction in sodium output, which parallels that of the intestinal output[100,107-114]. Magnesium balance has not been changed by octreotide[107,112]. Octreotide does not significantly change total energy[110,113,114] or nitrogen absorption[107,109,112-114]. Fat absorption may be unchanged [112-114] or reduced[107] as might be predicted from a reduction in pancreaticobiliary secretion. The effect of octreotide is maintained in the long-term[107,109,110,112,114]. Somatostatin and octreotide both reduce the output from a high fistula and appear to accelerate the rate of spontaneous closure[115,116].
Steroids/fludrocortisone/desmopressin Mineralocorticoids (e.g. 2 mg oral fludrocortisone or 2 mg intravenous d-aldosterone) [117-119] or high dose hydrocortisone[120] can occasionally reduce the stomal output in patients with a retained ileum. Desmopressin (an analogue of antidiuretic hormone) has no effect upon ileal fluid or electrolyte loss in man[121]. Some patients cannot be maintained with any oral regimens (usually if jejunal length is less than 100 cm) and need regular parenteral saline supplements. Most such patients also need oral or parenteral nutritional supplements, but a few need only one or two litres of parenteral (or subcutaneous) saline daily, often with added magnesium sulphate (4-12 mmol).
Magnesium deficiency occurs partly because its main absorptive sites have been removed (distal small bowel and colon)[122]. However the degree of malabsorption of magnesium does not correlate with the residual length of jejunum[42,123]. Fatty acids derived either from digestion of dietary fat or from bacterial fermentation of malabsorbed carbohydrate, combine with magnesium, calcium and zinc so preventing absorption and increasing faecal or stomal losses[124]. Another reason particularly in patients with a jejunostomy is that salt and water depletion causes secondary hyperaldosteronism, which increases renal magnesium excretion[125,126].
The clinical syndrome of magnesium deficiency in man includes fatigue, depression, jerky and weak muscles, ataxia, athetoid movements, cardiac arrhythmia’s and, if severe, convulsions[127-130]. The occurrence of carpopedal spasm, positive Chvostek and Trousseau’s signs generally occur if there is a concomitant hypocalcaemia[128,129,131]. Low serum magnesium levels are more common in patients with a jejunostomy (70%) than patients with a retained colon (40%)[25]. Hypomagnesaemia can perpetuate itself as it reduces both the secretion and function of parathormone[132]. Thus parathormone cannot promote magnesium absorption in the ascending limb of the loop of Henle or activate renal 1 alpha-hydroxylase, which catalyses the formation of 1, 25 hydroxy-vitamin D. The failure to make 1, 25 hydroxy-vitamin D results in reduced intestinal magnesium and calcium absorption[133].
Dehydration and sodium depletion, which will cause secondary hyperaldosteronism (which leads to renal magnesium loss), must first be treated. A diet relatively low in fat reduces stool/stomal magnesium losses especially in patients with a retained colon[124,134]. Various magnesium salts have been given as a treatment orally: magnesium sulphate, chloride, hydroxide, acetate, carbonate, gluconate, lactate, citrate, aspartate, pyroglutamate, oxide (magnesia) and diglycinate. Most magnesium salts are poorly absorbed and may worsen diarrhoea/stomal output. Magnesium acetate has been shown to cause less diarrhoea than magnesium gluconate[135]. Magnesium oxide is commonly given and contains more elemental magnesium than the other salts, is insoluble in water and alcohol but soluble in dilute acid. In the stomach it is converted to magnesium chloride. It is given as gelatine capsules of 4 mmol magnesium oxide (160 mg of MgO) to a total of 12-24 mmol•d¯¹. Magnesium oxide is usually given at night when intestinal transit is assumed to be slowest and hence there is more time for absorption. Magnesium diglycinate (chelate) is absorbed as well as magnesium oxide as an intact dipeptide in the proximal jejunum and it results in the passage of fewer stools, after an ileal resection, than magnesium oxide[136].
As hypomagnesaemia will have caused both a failure of parathormone release and a resistance to its action, 1-alpha hydroxy-cholecalciferol cannot be made in the kidney in adequate amounts. Thus if oral magnesium supplements do not bring the magnesium level into the normal range, oral 1-alpha hydroxy-cholecalciferol in a gradually increasing dose (every 2-4 wk) of 1-9 μg•d¯¹ will improve magnesium balance in patients, at least in those with a retained colon[137,138]. This action is by increasing both intestinal and renal magnesium absorption[138]. Occasionally magnesium is given as an intravenous or subcutaneous infusion.
Undernutrition only becomes apparent slowly and should be prevented from occurring by predicting its likelihood and from knowledge of the residual length of bowel remaining (Table 4). All patients who can be maintained on an oral diet need to consume more energy than normal subjects because 50% or more of the energy from the diet may be malabsorbed. Patients can achieve this by eating more high-energy food, having oral sip-feeds, or high energy enteral feeds given at night through a nasogastric or gastrostomy tube. There are rarely any problems inserting a percutaneous endoscopic gastrostomy (PEG) into patients with Crohn’s disease providing that there is no distal obstruction[136]. Once weight is regained, a nocturnal feed can be reduced or stopped and sip-feeds during the day may be adequate. Only if these measures fail and the patient continues to lose weight, or fails to regain lost weight, is parenteral nutrition given. Parenteral nutrition may only be needed for a few weeks or months before oral supplements are adequate.
| Jejunum-colon | Jejunostomy | |
| Presentation | gradual, diarrhoea and undernutrition | acute fluid losses |
| Water, sodium and | uncommon in the | common |
| magnesium depletion | long-term | |
| Nutrient malabsorption | common* | very common |
| D(-)lactic acidosis | occasionally | none |
| Renal stones (calcium oxalate) | 25% | none |
| Gallstones (pigment) | 45% | 45% |
| Adaptation | functional adaptation | no evidence |
| Social problems | diarrhoea dehydration dependency on treatment | high stomal output |
In the long-term parenteral nutrition is needed if a patient absorbs less than one-third of the oral energy intake[38,74], if there are high-energy requirements and absorption is 30%-60% or if increasing the oral/enteral nutrient intake causes a socially unacceptably large volume of stomal output or diarrhoea. In addition to consuming a high energy diet the dietary advice given to the two types of patient is different.
Jejunum-colon Unabsorbed long chain fatty acids in the colon reduce the transit time[139] and reduce water and sodium absorption[140] somaking diarrhoea worse. In addition they are toxic to bacteria so reduce carbohydrate fermentation[141]. They bind to calcium and magnesium increasing stool losses, and they increase oxalate absorption so predisposing to the formation of renal stones (see below). Theoretically, a low fat diet is ideal for patients after a small bowel resection[142], however in practice it is hard to implement. Fat yields twice as much energy as a comparable weight of carbohydrate or protein and makes food palatable. A high carbohydrate/low fat diet involves eating a large volume of food. A low fat diet may increase calcium, magnesium and zinc absorption[124] but makes essential fatty acid deficiency more likely[143]. Sunflower oil may be rubbed onto the skin to ensure adequate amounts of essential fatty acids enter the body[144]. If a diet is high in monosaccharides D (-) lactic acidosis may occur (see below). Medium chain triglycerides are an alternative source of energy and are absorbed from the small and large bowel[145-148]. In order to increase the energy absorption, reduce the risk of renal stones and D (-) lactic acidosis, patients with a retained colon need a large totalenergy intake with a diet high in carbohydrate (polysaccharides)[29], but not increased in fat (long chain triglycerides) and low in oxalate.
Jejunostomy Patients with a jejunostomy absorb a constant proportion of the nitrogen, energy and fat from their diet[29,134,149]. Increasing fat in the diet, increases energy density, keeps the diet osmolality low, increases palatability and provides essential fatty acids[143]. It does raise fat excretion but does not usually increase stomal output, nor make the output offensive[29,134,149]. Two studies have shown fatty acids to increase the stomal output of divalent cations[124,150] and two others found no such effect[134,149]. There is no advantage in giving a diet of small molecules (e.g. an elemental diet), which causes a feed to be hyper-osmolar[149] and usually contains little sodium, so stomal losses of water and sodium from the stoma increase. A peptide diet has a relatively high osmolality and thus can increase stomal output[151]. Little advantage comes from taking a diet of water-soluble medium chain triglycerides in place of normal fat[148]. The addition of glutamine (15 gm) to a litre of rehydration solution in patients with jejunostomy resulted in no additional benefit in terms of water or sodium absorption[152]. The fibre content of the diet plays only a minor role in determining jejunal output[93]. As the stomal sodium losses are about 100 mmol•L¯¹ any diet will need added sodium chloride. These patients need a diet of high energy (carbohydrate or lipid) in which the osmolality is kept low by using large molecules (polysaccharides, protein and triglycerides)[149,150] and to which sodium chloride is added to give the meal/liquid feed a total sodium concentration of 90-120 mmol•L¯¹ and an osmolality of about 300 mmol•L¯¹.
Conjugated bile acid treatment As fat malabsorption in both types of patient with a short bowel may partly be due to bile acid depletion, bile salts have been tried as a treatment. Cholylsarcosine, a synthetic bile acid resistant to bacterial deconjugation and dehydroxylation and which does not itself cause colonic secretion so does not cause diarrhoea. When 4 grams are taken three times a day, there is a variable improvement in fat and calcium absorption in patients with and without a retained functioning colon; however it may cause nausea[153-154].
Other vitamin and mineral deficiencies Both groups of patient have had more than 60 cm of their terminal ileum removed, and need long-term hydroxycobalamin injections [11,12,57]. Patients receiving parenteral nutrition are commonly selenium deficient[155-157] and need larger amounts of selenium than are required in the diet of normal subjects[157] this suggests a loss of selenium from the gastrointestinal secretions. In patients with a jejunostomy there is a reduction in selenium absorption, which relates to the length of remaining jejunum[158]. The kidney can conserve selenium but this may not be adequate and selenium deficiency is common. This causes weak muscles and a dilated cardiomyopathy[159]. Zinc deficiency is not common unless stool volumes are large[160]. There may be impairment of absorption of the fat-soluble vitamins A D E K and essential fatty acids. Essential fatty acid deficiency can be treated by rubbing sunflower oil into the skin[144].
The oral intake determines the amount of stool passed. Diarrhoea, which may severely restrict a patient’s life style, can be reduced by limiting food intake, but this may increase the problems of undernutrition. Rarely a patient needs parenteral nutrition to allow them to eat less and so reduce the diarrhoea.
Diarrhoea may be treated with loperamide 2-8 mg given half an hour before food in the same way as for patients with a jejunostomy and occasionally codeine phosphate is also added (30-60 mg half an hour before food). If less than 100 cm terminal ileum has been resected bile salt malabsorption may contribute to the diarrhoea and may be helped by cholestyramine, which has the additional advantage of reducing oxalate absorption, but may be detrimental by reducing fat absorption and by further reducing the bile salt pool[58]. Although a gastric anti-secretory drug may reduce diarrhoea shortly after surgery, they are not usually effective in the long-term.
In addition to the many common general medical causes of confusion (e.g. hypoxia, hepatic, renal or cardiac failure, sepsis, hypoglycaemia, alcohol or other drugs) other specific causes should be sought in a patient with a short bowel. Hypomagnesaemia may cause mild confusion when the serum magnesium level is very low (less than 0.2 mmol•L¯¹). Thiamine deficiency can cause a Wernicke/Korsik off psychosis, which responds rapidly to large regular doses of thiamine. Two other specific causes in patients with a short bowel are D(-) lactic acidosis and hyperammonaemia. D(-) lactic acidosis is only seen in patients with a short bowel and a preserved colon. Lactic acid produced and utilized by mammalian cells is the L(+) isomer but colonic bacteria produce both the L(+) and the D(-) isomers. In rare circumstances the colonic flora may rapidly degrade a surplus of easily fermentable carbohydrate (mono and oligosaccharides and rapidly digestible starch[161] to D(-) lactate. A reduction in colonic gram-negative anaerobes and a predominance of gram-positive anaerobes, especially Lactobacillus, Eubacterium, and Bifidobacterium has been found[162]. D(-) lactate is absorbed and cannot be easily metabosised. It causes ataxia, blurred vision, ophthalmoplegia and nystagmus, slurred speech, aggressiveness, inappropriate behaviour, and stupor, which may progress to coma[163]. It is suspected when a patient is found to have a metabolic acidosis with a large anion gap, and confirmed by increased concentrations of D (-) lactate in blood and urine. The diet is changed so that simplecarbohydrates (mono and oligosaccharides)[164] are restricted and more slowly digestible polysaccharides (starch) encouraged and thiamine may be given. Broad-spectrum antibiotics (neomycin or vancomycin) can be given to reduce the bacterial production of lactate. Medium chain triglycerides may be omitted[165]. If very severe the patient may need to fast and be given parenteral nutrition.
Confusion may occur in both types of patients with a short bowel due to hyperammonaemia, this results because ammonia cannot be detoxified. The small amount of intestine remaining cannot manufacture adequate citrulline to detoxify ammonia in the urea cycle. The increase in blood ammonia is a problem if there is concomitant renal impairment, as the excess ammonia cannot be excreted. By giving arginine (an intermediary in the urea cycle) the hyperammonaemia can be corrected[166,167].
Omeprazole can be absorbed in the duodenum/upper jejunum and only if less than 50 cm jejunum remains are problems likely to occur. Many drugs will be incompletely absorbed by patients with a short bowel and may be needed in much higher amounts than usual (e.g. thyroxine, warfarin[168], and digoxin[169]) or may need to be given intravenously. As the enterohepatic circulation, round which loperamide circulates, is disrupted, higher doses than usual may need to be given.
Gallstones are common (45%) in both types of patient and are more common in men[25]. It was originally thought that gallstones in this circumstance were due to deposition of cholesterol because of a depleted bile-salt pool. However, the gallstones tend to contain calcium bilirubinate and these probably result from gallbladder stasis with the consequent formation of biliary sludge. These stones may appear calcified on an abdominal radiograph[170]. Calcium bilirubinate crystals, within biliary sludge, are more commonly found in men than women[171]. The reasons for the formation of biliary sludge include, abdominal surgery, ileal resection, Crohn’s disease, rapid weight loss, drug therapy (e.g. octreotide) and altered gastrointestinal transit and flora. Disturbed cholesterol or bilirubin metabolism, and changes in nucleation may also be important[172]. There are many therapies proposed to prevent the formation of gallstones. Cholecystokinin (CCK) injections have been successful[173]. Periodic pulsed infusions of amino acids or small amounts of enteral feed stimulate endogenous CCK release, cause gallbladder contraction and thus prevent gallbladder stasis[174]. Non-steroidal anti-inflammatory drugs may prevent gallstones by decreasing the mucin glycoprotein involved in nucleation[175], or by a prokinetic effect (via leukotrienes) on the gallbladder[176]. Ursodeoxycholic acid (UDCA) causes bile to become richer in glycine or taurine conjugates of UDCA and this results in a marked reduction in cholesterol crystallisation. This may be due to a reduction in some crystallisation promoting factors (e.g. aminopeptidase N, haptoglobin and some immunoglobulins)[177,178]. Reducing the amount of the more lithogenic secondary bile acids could be achieved by either increasing intestinal transit (e.g. cisapride[179]) or by inhibiting bowel bacteria (e.g. metronidazole)[180]. Some units advocate prophylactic cholecystectomy whenever a large intestinal resection is performed[181].
Patients with a retained colon have a 25% chance of developing symptomatic calcium oxalate renal stones[25]. Oxalate is a metabolic end product that cannot be metabolised in man. Under normal circumstances, most urinary oxalate derives from the metabolism of amino acids (mainly glycine) and of ascorbic acid. Less than 10% is derived from dietary oxalate[182]. However after an ileal resection there is increased colonic absorption of dietary oxalate especially by the distal colon[183].
There are many factors contributing to calcium oxalate renal stone formation[184].
Fat malabsorption Increasing the dietary intake of lipid results in increasing enteric hyperoxaluria[185]. Fat malabsorption leads to calcium soap formation and calcium malabsorption and to oxalate being unbound and thus easily absorbable. Colonic oxalate absorption is increased by low dietary calcium, hyperparathyroidism, and vitamin D administration, all of which reduce calcium concentrations in the colon and thus the extent to which oxalate is bound to calcium in the gut lumen.
Increased colonic permeability Unabsorbed bile acids increase colonic permeability to oxalate. Chenodeoxycholate, infused into the colon, increased oxalate absorption five-fold[186]. Cholestyramine taken orally decreased oxalate absorption[187,188].
Reduced bacterial breakdown of oxalate An anaerobic bacteria (Oxalobacter formigenes) within the colon degrades oxalate to carbon dioxide and formate[189]. A reduction in its amount could contribute to increased oxalate absorption. It is cultured less frequently from the stools of patients with Crohn’s disease or steatorrhoea[190]. Its growth is inhibited by low bile acid concentrations as occur in patients with a short bowel and retained colon[191].
Vitamin deficiency Pyridoxine deficiency may increase the metabolic formation of oxalate and/or the ileal absorption[192,193]. Thiamine deficiency may increase oxalate production. In contrast ascorbic acid deficiency decreases oxalate absorption[194].
Hypocitraturia Citrate prevents nucleation, the first step in stone formation. Hypocitraturia is common in patients with malabsorption or jejunoileal bypass[195-197]. Citrate excretion and urine pH are reduced by systemic acidosis, including that caused by gastrointestinal bicarbonate wastage, and by hypomagnesaemia. Hypocitraturia in patients with malabsorption can be corrected by oral citrate supplementation and magnesium injections[196].
Type of oxalate ingested Sodium oxalate as found in tea is more readily absorbed that calcium oxalate in most foods.
To prevent calcium oxalate renal stone formation, patients should avoid dehydration and take a diet low in oxalate. A low oxalate diet means avoiding foods such as spinach, rhubarb, beetroot, nuts, chocolate, tea, wheat bran, and strawberries[184]. Fat restriction is theoretically good but may not be desirable for nutritional reasons. Substitution of medium chain triglycerides may be effective in reducing oxalate absorption[198]. Restriction of dietary oxalate and fat intake reduce urinary oxalate excretion[188,199-201]. Dietary calcium must not be restricted but must be increased. Calcium salts reduce absorption of dietary oxalate in patients with ileal resection or jejunoileal bypass[202-209]. Calcium-containing organic marine hydrocolloid decreases oxalate absorption without increasing calcium absorption[206]. Binding bile salts with cholestyramine has variable effects. It binds oxalate in vitro [210] and some studies showed it to decrease urinary oxalate excretion[187,210], however, other studies have shown no benefit[195,202] or even increased urinary oxalate excretion[211].
Aluminium salts can bind oxalate in vitro[205]. There was a halving of urinary oxalate excretion after oral aluminium hydroxide had been given to 4 patients with enteric hyperoxaluria[212], but no such reduction was observed in another study[211].
Most long-term patients with a short bowel have a body mass index within the normal range and most are at full time work or looking after the home and family unaided[25]. Both groups may have diarrhoea and in those with a colon the diarrhoea is malodorous and bulky due to steatorrhoea.
The effluent from a small-bowel stoma, unlike from a colostomy, is not offensive but sometimes is a large volume (e.g. 3 or more litres/24 h). The bag then has to be emptied frequently and, if it becomes overfull, the adhesive flange may separate from the skin with embarrassing leakage of fluid, and with the likelihood of skin soreness. A patient with no remaining jejunum and a preserved colon can survive without parenteral nutrition but their quality of life is poor[35].
New treatments aim to increase the absorptive function of the remaining gut. As plasma levels of GLP-2, which causes villus growth, and peptide YY, which slows upper gastrointestinal transit, are low in patients with a jejunostomy, treatments to correct these are being tried. GLP-2 gave a small increase in nutrient absorption[213]. Peptide YY agonists are available but have not yet been tried [214]. Other treatments that may increase structural adaptation include epidermal growth factor[215,216], colostrums and aminoguanidine[217]). Studies using growth hormone, glutamine and fibre have been disappointing[218,219]. Attempts are being made to replace colonic mucosa with small intestinal mucosa to increase absorption[220]. Other areas of importance are in the prevention of gallstones after an intestinal resection and randomised placebo controlled trials using prophylactic antibiotics, ursodeoxycholic acid and cholecystokinin are awaited. An oral nutrient solution containing 100 mmol/litre sodium and having an osmolality of 300 mmol•L¯¹ has yet to be commercially manufactured as an ideal nutrient solution to give to patients with a jejunostomy or high output ileostomy.
Small bowel transplantation is becoming a more successful operation, however few patients are suitable for this (no venous access or parenteral nutrition induced severe liver disease) and even when tacrolimus is used for immunosuppression only 40%-47% of patients will be alive 3 years later and 29%-38% will have a functioning graft[221,222]. 78% of those who survive with the transplant are able to stop parenteral nutrition. These figures contrast to those for patients on home parenteral nutrition for non-neoplastic reasons who can expect to have a relatively good quality of life good and a survival rate of 70% at 3 years[223,224].
Edited by Rampton DS and Pan BR
| 1. | Nightingale JMD. Introduction. Definition and classification of intestinal failure. In Intestinal Failure. Nightingale JMD (Ed). P xix -xx. Greenwich Medical Media Limited. 2001;. |
| 2. | Conn JH, Chavez CM, Fain WR. The short bowel syndrome. Ann Surg. 1972;175:803-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bryant J. Observations upon the growth and length of the human intestine. Am J Med Sci. 1924;167:499-520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | UNDERHILL BM. Intestinal length in man. Br Med J. 1955;2:1243-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 77] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Backman L, Hallberg D. Small-intestinal length. An intraoperative study in obesity. Acta Chir Scand. 1974;140:57-63. [PubMed] |
| 6. | Slater G, Aufses AH. Small bowel length in Crohn's disease. Am J Gastroenterol. 1991;86:1037-1040. [PubMed] |
| 7. | Schalamon J, Schober PH, Gallippi P, Matthyssens L, Höllwarth ME. Congenital short-bowel; a case study and review of the literature. Eur J Pediatr Surg. 1999;9:248-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Nightingale JM, Lennard-Jones JE. Adult patients with a short bowel due to Crohn's disease often start with a short normal bowel. Eur J Gastroenterol Hepatol. 1995;7:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | SINGLETON AO, REDMOND DC, MCMURRAY JE. ILEOCECAL RESECTION AND SMALL BOWEL TRANSIT AND ABSORPTION. Ann Surg. 1964;159:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Summers RW, Kent TH, Osborne JW. Effects of drugs, ileal obstruction, and irradiation on rat gastrointestinal propulsion. Gastroenterology. 1970;59:731-739. [PubMed] |
| 11. | BOOTH CC, MOLLIN DL. The site of absorption of vitamin B12 in man. Lancet. 1959;1:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 113] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Thompson WG, Wrathell E. The relation between ileal resection and vitamin B12 absorption. Can J Surg. 1977;20:461-464. [PubMed] |
| 13. | Nygaard K. Resection of the small intestine in rats. 3. Morphological changes in the intestinal tract. Acta Chir Scand. 1967;133:233-248. [PubMed] |
| 14. | Nygaard K. Resection of the small intestine in rats. IV. Adaptation of gastro-intestinal motility. Acta Chir Scand. 1967;133:407-416. [PubMed] |
| 15. | ALTHAUSEN TL, DOIG RK, UYEYAMA K, WEIDEN S. Digestion and absorption after massive resection of the small intestine. II. Recovery of the absorptive function as shown by intestinal absorption tests in two patients and a consideration of compensatory mechanisms. Gastroenterology. 1950;16:126-139. [PubMed] |
| 16. | Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967;32:139-149. [PubMed] |
| 17. | Weinstein LD, Shoemaker CP, Hersh T, Wright HK. Enhanced intestinal absorption after small bowel resection in man. Arch Surg. 1969;99:560-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 75] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Dowling RH, Booth CC. Functional compensation after small-bowel resection in man. Demonstration by direct measurement. Lancet. 1966;2:146-147. [PubMed] |
| 19. | Cosnes J, Carbonnel F, Beaugerie L, Ollivier JM, Parc R, Gendre JP, Le Quintrec Y. Functional adaptation after extensive small bowel resection in humans. Eur J Gastroenterol Hepatol. 1994;6:197-202. [DOI] [Full Text] |
| 20. | Fich A, Steadman CJ, Phillips SF, Camilleri M, Brown ML, Haddad AC, Thomforde GM. Ileocolonic transit does not change after right hemicolectomy. Gastroenterology. 1992;103:794-799. [PubMed] |
| 21. | Phillips SF, Giller J. The contribution of the colon to electrolyte and water conservation in man. J Lab Clin Med. 1973;81:733-746. [PubMed] |
| 22. | Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74:698-703. [PubMed] |
| 23. | Ladefoged K, Olgaard K. Fluid and electrolyte absorption and renin-angiotensin-aldosterone axis in patients with severe short-bowel syndrome. Scand J Gastroenterol. 1979;14:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Ladefoged K, Olgaard K. Sodium homeostasis after small-bowel resection. Scand J Gastroenterol. 1985;20:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Nightingale JM, Lennard-Jones JE, Gertner DJ, Wood SR, Bartram CI. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33:1493-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 203] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Fawcett SL, Cousins RJ. Magnesium poisoning following enema of epsom salt solution. J Am Med Assoc. 1943;123:1028-1029. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Hylander E, Ladefoged K, Jarnum S. Calcium absorption after intestinal resection. The importance of a preserved colon. Scand J Gastroenterol. 1990;25:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500-1507. [PubMed] |
| 29. | Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet. 1994;343:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 203] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut. 1998;43:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Nightingale JM, Kamm MA, van der Sijp JR, Morris GP, Walker ER, Mather SJ, Britton KE, Lennard-Jones JE. Disturbed gastric emptying in the short bowel syndrome. Evidence for a 'colonic brake'. Gut. 1993;34:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Miazza BM, Al-Mukhtar MY, Salmeron M, Ghatei MA, Felce-Dachez M, Filali A, Villet R, Wright NA, Bloom SR, Crambaud JC. Hyperenteroglucagonaemia and small intestinal mucosal growth after colonic perfusion of glucose in rats. Gut. 1985;26:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | JORDAN PH, STUART JR, BRIGGS JD. Radical small-bowel resection; report of two cases. Am J Dig Dis. 1958;3:823-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | ANDERSON CM. LONG-TERM SURVIVAL WITH SIX INCHES OF SMALL INTESTINE. Br Med J. 1965;1:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | KINNEY JM, GOLDWYN RM, BARR JS, MOORE FD. Loss of the entire jejunum and ileum, and the ascending colon. Management of a patient. JAMA. 1962;179:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Carbonnel F, Cosnes J, Chevret S, Beaugerie L, Ngô Y, Malafosse M, Parc R, Le Quintrec Y, Gendre JP. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. JPEN J Parenter Enteral Nutr. 1996;20:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Gouttebel MC, Saint-Aubert B, Astre C, Joyeux H. Total parenteral nutrition needs in different types of short bowel syndrome. Dig Dis Sci. 1986;31:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Nightingale JM, Lennard-Jones JE, Walker ER, Farthing MJ. Jejunal efflux in short bowel syndrome. Lancet. 1990;336:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 133] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Simons BE, Jordan GL. Massive bowel resection. Am J Surg. 1969;118:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Nightingale JM, Kamm MA, van der Sijp JR, Ghatei MA, Bloom SR, Lennard-Jones JE. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the 'colonic brake' to gastric emptying. Gut. 1996;39:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 241] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Nightingale JMD. Physiological consequences of major small intestinal resection in man and their treatment. MD Thesis, University of London. 1993;190-195. |
| 43. | FREDERICK PL, SIZER JS, OSBORNE MP. RELATION OF MASSIVE BOWEL RESECTION TO GASTRIC SECRETION. N Engl J Med. 1965;272:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | SHELTON EL, BLAINE MH. Massive small bowel resection in postgastrectomy patients report of 2 cases. Tex State J Med. 1954;50:96-101. [PubMed] |
| 45. | CRAIG TV, STEWART WR. Massive bowel resection in a patient with 75 per cent gastrectomy. Surgery. 1960;48:678-681. [PubMed] |
| 46. | Straus E, Gerson CD, Yalow RS. Hypersecretion of gastrin associated with the short bowel syndrome. Gastroenterology. 1974;66:175-180. [PubMed] |
| 47. | Williams NS, Evans P, King RF. Gastric acid secretion and gastrin production in the short bowel syndrome. Gut. 1985;26:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Temperley JM, Stagg BH, Wyllie JH. Disappearance of gastrin and pentagastrin in the portal circulation. Gut. 1971;12:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Becker HD, Reeder DD, Thompson JC. Extraction of circulating endogenous gastrin by the small bowel. Gastroenterology. 1973;65:903-906. [PubMed] |
| 50. | Fielding JF, Cooke WT, Williams JA. Gastric acid secretion in Crohn's disease in relation to disease activity and bowel resection. Lancet. 1971;1:1106-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Fitzpatrick WJ, Zentler-Munro PL, Northfield TC. Ileal resection: effect of cimetidine and taurine on intrajejunal bile acid precipitation and lipid solubilisation. Gut. 1986;27:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Windsor CW, Fejfar J, Woodward DA. Gastric secretion after massive small bowel resection. Gut. 1969;10:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | O'Keefe SJ, Haymond MW, Bennet WM, Oswald B, Nelson DK, Shorter RG. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterology. 1994;107:379-388. [PubMed] |
| 54. | Barbezat GO, Hansen JD. The exocrine pancreas and protein-calorie malnutrition. Pediatrics. 1968;42:77-92. [PubMed] |
| 55. | Kotler DP, Levine GM. Reversible gastric and pancreatic hyposecretion after long-term total parenteral nutrition. N Engl J Med. 1979;300:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Remington M, Fleming CR, Malagelada JR. Inhibition of postprandial pancreatic and biliary secretion by loperamide in patients with short bowel syndrome. Gut. 1982;23:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | BOOTH CC. The metabolic effects of intestinal resection in man. Postgrad Med J. 1961;37:725-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62:918-934. [PubMed] |
| 59. | Booth CC, Alldis D, Read AE. Studies on the site of fat absorption: 2 Fat balances after resection of varying amounts of the small intestine in man. Gut. 1961;2:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Goodlad RA, Nightingale JMD, Playford RJ. Intestinal adaptation. In Intestinal Failure. Nightingale JMD (Ed). P243-260. Greenwich Medical Media Limited. 2001;. |
| 61. | Crenn P, Morin MC, Joly F, Penven S, Thuillier F, Messing B. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut. 2004;53:1279-1286. [PubMed] |
| 62. | Nightingale J. Short bowel, short answer. Gut. 1999;45:478-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | PORUS RL. EPITHELIAL HYPERPLASIA FOLLOWING MASSIVE SMALL BOWEL RESECTION IN MAN. Gastroenterology. 1965;48:753-757. [PubMed] |
| 64. | De Francesco A, Malfi G, Delsedime L, David E, Pera A, Serra R, Avagnina S, Boggio Bertinet D, Delle Piane D, De Magistris A. Histological findings regarding jejunal mucosa in short bowel syndrome. Transplant Proc. 1994;26:1455-1456. [PubMed] |
| 65. | Jeppesen PB, Hartmann B, Thulesen J, Hansen BS, Holst JJ, Poulsen SS, Mortensen PB. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut. 2000;47:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Gouttebel MC, Saint Aubert B, Colette C, Astre C, Monnier LH, Joyeux H. Intestinal adaptation in patients with short bowel syndrome. Measurement by calcium absorption. Dig Dis Sci. 1989;34:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 392] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 68. | Hill GL, Mair WS, Goligher JC. Impairment of 'ileostomy adaptation' in patients after ileal resection. Gut. 1974;15:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Adrian TE, Savage AP, Fuessl HS, Wolfe K, Besterman HS, Bloom SR. Release of peptide YY (PYY) after resection of small bowel, colon, or pancreas in man. Surgery. 1987;101:715-719. [PubMed] |
| 71. | Nightingale JM, Bartram CI, Lennard-Jones JE. Length of residual small bowel after partial resection: correlation between radiographic and surgical measurements. Gastrointest Radiol. 1991;16:305-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 73. | Nightingale JM, Walsh N, Bullock ME, Wicks AC. Three simple methods of detecting malnutrition on medical wards. J R Soc Med. 1996;89:144-148. [PubMed] |
| 74. | Rodrigues CA, Lennard-Jones JE, Thompson DG, Farthing MJ. Energy absorption as a measure of intestinal failure in the short bowel syndrome. Gut. 1989;30:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | BORGSTROM B, DAHLQVIST A, LUNDH G, SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957;36:1521-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 644] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 76. | Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11:503-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 275] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Vantrappen GR, Peeters TL, Janssens J. The secretory component of the interdigestive migrating motor complex in man. Scand J Gastroenterol. 1979;14:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 135] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Whang R, Whang DD, Ryan MP. Refractory potassium repletion. A consequence of magnesium deficiency. Arch Intern Med. 1992;152:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Solomon R. The relationship between disorders of K+ and Mg+ homeostasis. Semin Nephrol. 1987;7:253-262. [PubMed] |
| 80. | Newton CR, Drury P, Gonvers JJ, McIntyre P, Preston DM, Lennard-Jones JE. Incidence and treatment of sodium depletion in ileostomists. Scand J Gastroenterol Suppl. 1982;74:159-160. [PubMed] |
| 81. | Kennedy HJ, Al-Dujaili EA, Edwards CR, Truelove SC. Water and electrolyte balance in subjects with a permanent ileostomy. Gut. 1983;24:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Newton CR, Gonvers JJ, McIntyre PB, Preston DM, Lennard-Jones JE. Effect of different drinks on fluid and electrolyte losses from a jejunostomy. J R Soc Med. 1985;78:27-34. [PubMed] |
| 83. | Griffin GE, Fagan EF, Hodgson HJ, Chadwick VS. Enteral therapy in the management of massive gut resection complicated by chronic fluid and electrolyte depletion. Dig Dis Sci. 1982;27:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Nightingale JM, Lennard-Jones JE, Walker ER, Farthing MJ. Oral salt supplements to compensate for jejunostomy losses: comparison of sodium chloride capsules, glucose electrolyte solution, and glucose polymer electrolyte solution. Gut. 1992;33:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Rodrigues CA, Lennard JJE, Thompson DG, Farthing MJG. What is the ideal sodium concentration of oral rehydration solutions for short bowel patients. Clin Sci. 1988;74:69p. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Woolf GM, Miller C, Kurian R, Jeejeebhoy KN. Nutritional absorption in short bowel syndrome. Evaluation of fluid, calorie, and divalent cation requirements. Dig Dis Sci. 1987;32:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Olsen WA, Ingelfinger FJ. The role of sodium in intestinal glucose absorption in man. J Clin Invest. 1968;47:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Sladen GE, Dawson AM. Interrelationships between the absorptions of glucose, sodium and water by the normal human jejunum. Clin Sci. 1969;36:119-132. [PubMed] |
| 89. | Fordtran JS. Stimulation of active and passive sodium absorption by sugars in the human jejunum. J Clin Invest. 1975;55:728-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Avery ME, Snyder JD. Oral therapy for acute diarrhea. The underused simple solution. N Engl J Med. 1990;323:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Beaugerie L, Cosnes J, Verwaerde F, Dupas H, Lamy P, Gendre JP, Le Quintrec Y. Isotonic high-sodium oral rehydration solution for increasing sodium absorption in patients with short-bowel syndrome. Am J Clin Nutr. 1991;53:769-772. [PubMed] |
| 92. | Bodemar G, Sjödahl R. Rice and glucose oral rehydration solutions in patients with high ileostoma fluid output. Lancet. 1992;340:862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 93. | Newton CR. Effect of codeine phosphate, Lomotil, and Isogel on iileostomy function. Gut. 1978;19:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | King RF, Norton T, Hill GL. A double-blind crossover study of the effect of loperamide hydrochloride and codeine phosphate on ileostomy output. Aust N Z J Surg. 1982;52:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Tytgat GN, Huibregtse K. Loperamide and ileostomy output--placebo-controled double-blind crossover study. Br Med J. 1975;2:667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Tytgat GN, Huibregtse K, Dagevos J, van den Ende A. Effect of loperamide on fecal output and composition in well-established ileostomy and ileorectal anastomosis. Am J Dig Dis. 1977;22:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Remington M, Fleming CR, Malagelada JR. Inhibition of postprandial pancreatic and biliary secretion by loperamide in patients with short bowel syndrome. Gut. 1982;23:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Nightingale JM, Lennard-Jones JE, Walker ER. A patient with jejunostomy liberated from home intravenous therapy after 14 years; contribution of balance studies. Clin Nutr. 1992;11:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | O'Brien JD, Thompson DG, McIntyre A, Burnham WR, Walker E. Effect of codeine and loperamide on upper intestinal transit and absorption in normal subjects and patients with postvagotomy diarrhoea. Gut. 1988;29:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Rodrigues CA, Lennard-Jones JE, Thompson DG, Farthing MJ. The effects of octreotide, soy polysaccharide, codeine and loperamide on nutrient, fluid and electrolyte absorption in the short-bowel syndrome. Aliment Pharmacol Ther. 1989;3:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Osborne MP, Frederick PL, Sizer JS, Blair D, Cole P, Thum W. Mechanism of gastric hypersecretion following massive intestinal resection. Clinical and experimental observations. Ann Surg. 1966;164:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Aly A, Bárány F, Kollberg B, Monsén U, Wisén O, Johansson C. Effect of an H2-receptor blocking agent on diarrhoeas after extensive small bowel resection in Crohn's disease. Acta Med Scand. 1980;207:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Jacobsen O, Ladefoged K, Stage JG, Jarnum S. Effects of cimetidine on jejunostomy effluents in patients with severe short-bowel syndrome. Scand J Gastroenterol. 1986;21:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 104. | Nightingale JM, Walker ER, Farthing MJ, Lennard-Jones JE. Effect of omeprazole on intestinal output in the short bowel syndrome. Aliment Pharmacol Ther. 1991;5:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Jeppesen PB, Staun M, Tjellesen L, Mortensen PB. Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut. 1998;43:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 106. | Dharmsathaphorn K, Gorelick FS, Sherwin RS, Cataland S, Dobbins JW. Somatostatin decreases diarrhea in patients with the short-bowel syndrome. J Clin Gastroenterol. 1982;4:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Cooper JC, Williams NS, King RF, Barker MC. Effects of a long-acting somatostatin analogue in patients with severe ileostomy diarrhoea. Br J Surg. 1986;73:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Kusuhara K, Kusunoki M, Okamoto T, Sakanoue Y, Utsunomiya J. Reduction of the effluent volume in high-output ileostomy patients by a somatostatin analogue, SMS 201-995. Int J Colorectal Dis. 1992;7:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Shaffer JL, O’Hanrahan T, Rowntree S, Shipley K, Irving MH. Does somatostatin analogue (201-995) reduce high output stoma effluent A controlled trial. Gut. 1988;29:A1432-1433. |
| 110. | Nightingale JM, Walker ER, Burnham WR, Farthing MJ, Lennard-Jones JE. Octreotide (a somatostatin analogue) improves the quality of life in some patients with a short intestine. Aliment Pharmacol Ther. 1989;3:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Nightingale JM. The Sir David Cuthbertson Medal Lecture. Clinical problems of a short bowel and their treatment. Proc Nutr Soc. 1994;53:373-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Ladefoged K, Christensen KC, Hegnhøj J, Jarnum S. Effect of a long acting somatostatin analogue SMS 201-995 on jejunostomy effluents in patients with severe short bowel syndrome. Gut. 1989;30:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Léann M, de Montigny S, Mahé S, Thuillier F, Huneau JF, Tomé D, Rambaud JC, Messing B. Effect of octreotide on water and electrolytes losses, nutrient absorption and transit in short bowel syndrome. Eur J Gastroenterol Hepatol. 1993;5:817-22. |
| 114. | O'Keefe SJ, Peterson ME, Fleming CR. Octreotide as an adjunct to home parenteral nutrition in the management of permanent end-jejunostomy syndrome. JPEN J Parenter Enteral Nutr. 1994;18:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Nubiola P, Badia JM, Martinez-Rodenas F, Gil MJ, Segura M, Sancho J, Sitges-Serra A. Treatment of 27 postoperative enterocutaneous fistulas with the long half-life somatostatin analogue SMS 201-995. Ann Surg. 1989;210:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Scott NA, Finnegan S, Irving MH. Octreotide and postoperative enterocutaneous fistulae: a controlled prospective study. Acta Gastroenterol Belg. 1993;56:266-270. [PubMed] |
| 117. | GOULSTON K, HARRISON DD. EFFECT OF MINERALOCORTICOIDS ON THE SODIUM/POTASSIUM RATIOOF HUMAN ILEOSTOMY FLUID. Lancet. 1963;2:541-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | Levitan R, Goulston K. Water and electrolyte content of human ileostomy fluid after d-aldosterone administration. Gastroenterology. 1967;52:510-512. [PubMed] |
| 119. | Kramer P, Levitan R. Effect of 9- -fluorohydrocortisone on the ileal excreta of ileostomized subjects. Gastroenterology. 1972;62:235-241. [PubMed] |
| 120. | Feretis CB, Vyssoulis GP, Pararas BN, Nissiotis AS, Calaitzopoulos JD, Apostolidis NS, Golematis BC. The influence of corticosteroids on ileostomy discharge of patients operated for ulcerative colitis. Am Surg. 1984;50:433-436. [PubMed] |
| 121. | Sutters M, Carmichael DJ, Unwin RJ, Sozi C, Hunter M, Calam J, Lightman SL, Peart WS. 'Low sodium' diuresis and ileal loss in patients with ileostomies: effect of desmopressin. Gut. 1991;32:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 122. | Kayne LH, Lee DB. Intestinal magnesium absorption. Miner Electrolyte Metab. 1993;19:210-217. [PubMed] |
| 123. | Nightingale JMD. Management of a high output jejunostomy. In Intestinal Failure. Nightingale JMD (Ed). P375-392. Greenwich Medical Media Limited. 2001;. |
| 124. | Hessov I, Andersson H, Isaksson B. Effects of a low-fat diet on mineral absorption in small-bowel disease. Scand J Gastroenterol. 1983;18:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | HANNA S, MacINTYRE I. The influence of aldosterone on magnesium metabolism. Lancet. 1960;2:348-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | HORTON R, BIGLIERI EG. Effect of aldosterone on the metabolism of magnesium. J Clin Endocrinol Metab. 1962;22:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | VALLEE BL, WACKER WE, ULMER DD. The magnesium-deficiency tetany syndrome in man. N Engl J Med. 1960;262:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | HANNA S, HARRISON M, MACINTYRE I, FRASER R. The syndrome of magnesium deficiency in man. Lancet. 1960;2:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | BOOTH CC, BABOURIS N, HANNA S, MACINTYRE I. Incidence of hypomagnesaemia in intestinal malabsorption. Br Med J. 1963;2:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 130. | Fleming CR, George L, Stoner GL, Tarrosa VB, Moyer TP. The importance of urinary magnesium values in patients with gut failure. Mayo Clin Proc. 1996;71:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Rude RK, Singer FR. Magnesium deficiency and excess. Annu Rev Med. 1981;32:245-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 118] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 132. | Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK. Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1, 25-dihydroxyvitamin D metabolism. J Clin Endocrinol Metab. 1991;73:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 104] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 133. | Zofková I, Kancheva RL. The relationship between magnesium and calciotropic hormones. Magnes Res. 1995;8:77-84. [PubMed] |
| 134. | Ovesen L, Chu R, Howard L. The influence of dietary fat on jejunostomy output in patients with severe short bowel syndrome. Am J Clin Nutr. 1983;38:270-277. [PubMed] |
| 135. | Kaufman SS, Loseke CA, Anderson JB, Murray ND, Vanderhoof JA, Young RJ. Magnesium acetate vs. magnesium gluconate supplementation in short bowel syndrome. J Pediatr Gastroenterol Nutr. 1993;16:104-105. [PubMed] |
| 136. | Schuette SA, Lashner BA, Janghorbani M. Bioavailability of magnesium diglycinate vs magnesium oxide in patients with ileal resection. JPEN J Parenter Enteral Nutr. 1994;18:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 137. | Selby PL, Peacock M, Bambach CP. Hypomagnesaemia after small bowel resection: treatment with 1 alpha-hydroxylated vitamin D metabolites. Br J Surg. 1984;71:334-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Fukumoto S, Matsumoto T, Tanaka Y, Harada S, Ogata E. Renal magnesium wasting in a patient with short bowel syndrome with magnesium deficiency: effect of 1 alpha-hydroxyvitamin D3 treatment. J Clin Endocrinol Metab. 1987;65:1301-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Spiller RC, Brown ML, Phillips SF. Decreased fluid tolerance, accelerated transit, and abnormal motility of the human colon induced by oleic acid. Gastroenterology. 1986;91:100-107. [PubMed] |
| 140. | Ammon HV, Phillips SF. Inhibition of colonic water and electrolyte absorption by fatty acids in man. Gastroenterology. 1973;65:744-749. [PubMed] |
| 141. | Knapp HR, Melly MA. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis. 1986;154:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 142. | Andersson H. The use of a low-fat diet in the symptomatic treatment of ileopathia. World Rev Nutr Diet. 1982;40:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 143. | Jeppesen PB, Christensen MS, Høy CE, Mortensen PB. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843. [PubMed] |
| 144. | Press M, Hartop PJ, Prottey C. Correction of essential fatty-acid deficiency in man by the cutaneous application of sunflower-seed oil. Lancet. 1974;1:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Zurier RB, Campbell RG, Hashim SA, Van Itallie TB. Use of medium-chain triglyceride in management of patients with massive resection of the small intestine. N Engl J Med. 1966;274:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 146. | Bochenek W, Rodgers JB, Balint JA. Effects of changes in dietary lipids on intestinal fluid loss in the short-bowel syndrome. Ann Intern Med. 1970;72:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 147. | Tandon RK, Rodgers JB, Balint JA. The effects of medium-chain triglycerides in the short bowel syndrome. Increased glucose and water transport. Am J Dig Dis. 1972;17:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 148. | Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut. 1998;43:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 149. | McIntyre PB, Fitchew M, Lennard-Jones JE. Patients with a high jejunostomy do not need a special diet. Gastroenterology. 1986;91:25-33. [PubMed] |
| 150. | Woolf GM, Miller C, Kurian R, Jeejeebhoy KN. Diet for patients with a short bowel: high fat or high carbohydrate. Gastroenterology. 1983;84:823-828. [PubMed] |
| 151. | Bosaeus I, Carlsson NG, Andersson H. Low-fat versus medium-fat enteral diets. Effects on bile salt excretion in jejunostomy patients. Scand J Gastroenterol. 1986;21:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 152. | Beaugerie L, Carbonnel F, Hecketsweiler B, Déchelotte P, Gendre JP, Cosnes J. Effects of an isotonic oral rehydration solution, enriched with glutamine, on fluid and sodium absorption in patients with a short-bowel. Aliment Pharmacol Ther. 1997;11:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 153. | Gruy-Kapral C, Little KH, Fordtran JS, Meziere TL, Hagey LR, Hofmann AF. Conjugated bile acid replacement therapy for short-bowel syndrome. Gastroenterology. 1999;116:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 154. | Heydorn S, Jeppesen PB, Mortensen PB. Bile acid replacement therapy with cholylsarcosine for short-bowel syndrome. Scand J Gastroenterol. 1999;34:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 155. | Davis AT, Franz FP, Courtnay DA, Ullrey DE, Scholten DJ, Dean RE. Plasma vitamin and mineral status in home parenteral nutrition patients. JPEN J Parenter Enteral Nutr. 1987;11:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 156. | Shenkin A, Fell GS, Halls DJ, Dunbar PM, Holbrook IB, Irving MH. Essential trace element provision to patients receiving home intravenous nutrition in the United Kingdom. Clin Nutr. 1986;5:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 157. | Rannem T, Ladefoged K, Hylander E, Hegnhøj J, Jarnum S. Selenium depletion in patients on home parenteral nutrition. The effect of selenium supplementation. Biol Trace Elem Res. 1993;39:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 158. | Rannem T, Hylander E, Ladefoged K, Staun M, Tjellesen L, Jarnum S. The metabolism of [75Se]selenite in patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 1996;20:412-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 159. | Arthur JR, Beckett GJ. New metabolic roles for selenium. Proc Nutr Soc. 1994;53:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 160. | Wolman SL, Anderson GH, Marliss EB, Jeejeebhoy KN. Zinc in total parenteral nutrition: requirements and metabolic effects. Gastroenterology. 1979;76:458-467. [PubMed] |
| 161. | No authors listed. The colon, the rumen, and D-lactic acidosis. Lancet. 1990;336:599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 162. | Stolberg L, Rolfe R, Gitlin N, Merritt J, Mann L, Linder J, Finegold S. d-Lactic acidosis due to abnormal gut flora: diagnosis and treatment of two cases. N Engl J Med. 1982;306:1344-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 101] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 163. | Dahlquist NR, Perrault J, Callaway CW, Jones JD. D-Lactic acidosis and encephalopathy after jejunoileostomy: response to overfeeding and to fasting in humans. Mayo Clin Proc. 1984;59:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 164. | Mayne AJ, Handy DJ, Preece MA, George RH, Booth IW. Dietary management of D-lactic acidosis in short bowel syndrome. Arch Dis Child. 1990;65:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Jover R, León J, Palazón JM, Domínguez JR. D-lactic acidosis associated with use of medium-chain triglycerides. Lancet. 1995;346:314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 166. | Yamada E, Wakabayashi Y, Saito A, Yoda K, Tanaka Y, Miyazaki M. Hyperammonaemia caused by essential aminoacid supplements in patient with short bowel. Lancet. 1993;341:1542-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 167. | Yokoyama K, Ogura Y, Kawabata M, Hinoshita F, Suzuki Y, Hara S, Yamada A, Mimura N, Nakayama M, Kawaguchi Y. Hyperammonemia in a patient with short bowel syndrome and chronic renal failure. Nephron. 1996;72:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 168. | Brophy DF, Ford SL, Crouch MA. Warfarin resistance in a patient with short bowel syndrome. Pharmacotherapy. 1998;18:646-649. [PubMed] |
| 169. | Ehrenpreis ED, Guerriero S, Nogueras JJ, Carroll MA. Malabsorption of digoxin tablets, gel caps, and elixir in a patient with an end jejunostomy. Ann Pharmacother. 1994;28:1239-1240. [PubMed] |
| 170. | Heaton KW, Read AE. Gall stones in patients with disorders of the terminal ileum and disturbed bile salt metabolism. Br Med J. 1969;3:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 134] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 171. | Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 311] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 172. | Byrne MF, Murray FE. Gallstones. In Intestinal Failure. Nightingale JMD (Ed). P 213-226.Greenwich Medical Media Limited. 2001;. |
| 173. | Doty JE, Pitt HA, Porter-Fink V, Denbesten L. Cholecystokinin prophylaxis of parenteral nutrition-induced gallbladder disease. Ann Surg. 1985;201:76-80. [PubMed] |
| 174. | Nealon WH, Upp JR, Alexander RW, Gomez G, Townsend CM, Thompson JC. Intravenous amino acids stimulate human gallbladder emptying and hormone release. Am J Physiol. 1990;259:G173-G178. [PubMed] |
| 175. | Lee SP, Carey MC, LaMont JT. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981;211:1429-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 167] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 176. | O'Donnell LJ, Wilson P, Guest P, Catnach SM, McLean A, Wickham JE, Fairclough PD. Indomethacin and postprandial gallbladder emptying. Lancet. 1992;339:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 177. | Portincasa P, van Erpecum KJ, Jansen A, Renooij W, Gadellaa M, vanBerge-Henegouwen GP. Behavior of various cholesterol crystals in bile from patients with gallstones. Hepatology. 1996;23:738-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 178. | Van Erpecum KJ, Stolk MF, van den Broek AM, Renooij W, van de Heijning BJ, van Berge Henegouwen GP. Bile concentration promotes nucleation of cholesterol monohydrate crystals by increasing the cholesterol concentration in the vesicles. Eur J Clin Invest. 1993;23:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 179. | Hussaini SH, Pereira SP, Dowling RH, Wass JA. Slow intestinal transit and gallstone formation. Lancet. 1993;341:638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 180. | Capron JP, Gineston JL, Herve MA, Braillon A. Metronidazole in prevention of cholestasis associated with total parenteral nutrition. Lancet. 1983;1:446-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 181. | Thompson JS. The role of prophylactic cholecystectomy in the short-bowel syndrome. Arch Surg. 1996;131:556-59; discussion 556-59;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 182. | Finch AM, Kasidas GP, Rose GA. Urine composition in normal subjects after oral ingestion of oxalate-rich foods. Clin Sci (. Lond). 1981;60:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 183. | Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol. 1994;5:1339-1343. [PubMed] |
| 184. | Tomson CRV. Nephrocalcinosis and nephrolithiasis. In Intestinal Failure. Nightingale JMD (Ed). P227-242. Greenwich Medical Media Limited. 2001;. |
| 185. | Earnest DL, Johnson G, Williams HE, Admirand WH. Hyperoxaluria in patients with ileal resection: an abnormality in dietary oxalate absorption. Gastroenterology. 1974;66:1114-1122. [PubMed] |
| 186. | Fairclough PD, Feest TG, Chadwick VS, Clark ML. Effect of sodium chenodeoxycholate on oxalate absorption from the excluded human colon--a mechanism for 'enteric' hyperoxaluria. Gut. 1977;18:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 187. | Smith LH, Fromm H, Hofmann AF. Acquired hyperoxaluria, nephrolithiasis, and intestinal disease. Description of a syndrome. N Engl J Med. 1972;286:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 166] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 188. | Chadwick VS, Modha K, Dowling RH. Mechanism for hyperoxaluria in patients with ileal dysfunction. N Engl J Med. 1973;289:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 154] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 189. | Argenzio RA, Liacoz JA, Allison MJ. Role of intestinal oxalate degrading bacteria in the pathogenesis of enteric hyperoxaluria (abstract). Gastroenterology. 1985;88:1309. |
| 190. | Allison MJ, Cook HM, Milne DB, Gallagher S, Clayman RV. Oxalate degradation by gastrointestinal bacteria from humans. J Nutr. 1986;116:455-460. [PubMed] |
| 191. | Argenzio RA, Liacos JA, Allison MJ. Intestinal oxalate-degrading bacteria reduce oxalate absorption and toxicity in guinea pigs. J Nutr. 1988;118:787-792. [PubMed] |
| 192. | Marangella M, Vitale C, Petrarulo M, Cosseddu D, Gallo L, Linari F. Pathogenesis of severe hyperoxalaemia in Crohn's disease-related renal failure on maintenance haemodialysis: successful management with pyridoxine. Nephrol Dial Transplant. 1992;7:960-964. [PubMed] |
| 193. | Farooqui S, Nath R, Thind SK, Mahmood A. Effect of pyridoxine deficiency on intestinal absorption of calcium and oxalate: chemical composition of brush border membranes in rats. Biochem Med. 1984;32:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 194. | Farooqui S, Thind SK, Nath R, Mahmood A. Intestinal absorption of oxalate in scorbutic and ascorbic acid supplemented guinea pigs. Acta Vitaminol Enzymol. 1983;5:235-241. [PubMed] |
| 195. | O'Leary JP, Thomas WC, Woodward ER. Urinary tract stone after small bowel bypass for morbid obesity. Am J Surg. 1974;127:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 196. | Rudman D, Dedonis JL, Fountain MT, Chandler JB, Gerron GG, Fleming GA, Kutner MH. Hypocitraturia in patients with gastrointestinal malabsorption. N Engl J Med. 1980;303:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 97] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 197. | Nicar MJ, Skurla C, Sakhaee K, Pak CY. Low urinary citrate excretion in nephrolithiasis. Urology. 1983;21:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 149] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 198. | Earnest DL, Williams HE, Admirand WH. A physicochemical basis for treatment of enteric hyperoxaluria. Trans Assoc Am Physicians. 1975;88:224-234. [PubMed] |
| 199. | Dobbins JW, Binder HJ. Importance of the colon in enteric hyperoxaluria. N Engl J Med. 1977;296:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 137] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 200. | Hofmann AF, Laker MF, Dharmsathaphorn K, Sherr HP, Lorenzo D. Complex pathogenesis of hyperoxaluria after jejunoileal bypass surgery. Oxalogenic substances in diet contribute to urinary oxalate. Gastroenterology. 1983;84:293-300. [PubMed] |
| 201. | Nordenvall B, Backman L, Burman P, Larsson L, Tiselius HG. Low-oxalate, low-fat dietary regimen in hyperoxaluria following jejunoileal bypass. Acta Chir Scand. 1983;149:89-91. [PubMed] |
| 202. | Caspary WF, Tönissen J, Lankisch PG. 'Enteral' hyperoxaluria. Effect of cholestyramine, calcium, neomycin, and bile acids on intestinal oxalate absorption in man. Acta Hepatogastroenterol (. Stuttg). 1977;24:193-200. [PubMed] |
| 203. | Barilla DE, Notz C, Kennedy D, Pak CY. Renal oxalate excretion following oral oxalate loads in patients with ileal disease and with renal and absorptive hypercalciurias. Effect of calcium and magnesium. Am J Med. 1978;64:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 101] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 204. | Hylander E, Jarnum S, Nielsen K. Calcium treatment of enteric hyperoxaluria after jejunoileal bypass for morbid obesity. Scand J Gastroenterol. 1980;15:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 205. | Laker MF, Hofmann AF. Effective therapy of enteric hyperoxaluria: in vitro binding of oxalate by anion-exchange resins and aluminum hydroxide. J Pharm Sci. 1981;70:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 206. | Lindsjö M, Fellström B, Ljunghall S, Wikström B, Danielson BG. Treatment of enteric hyperoxaluria with calcium-containing organic marine hydrocolloid. Lancet. 1989;2:701-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 207. | D'Cruz DP, Gertner DJ, Kasidas GP, Rampton DS, Rose GA, Samuell CT. Failure of allopurinol to modify urinary composition in enteric hyperoxaluria. Br J Urol. 1989;64:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 208. | Harper J, Mansell MA. Treatment of enteric hyperoxaluria. Postgrad Med J. 1991;67:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 209. | McLeod RS, Churchill DN. Urolithiasis complicating inflammatory bowel disease. J Urol. 1992;148:974-978. [PubMed] |
| 210. | Stauffer JQ, Humphreys MH, Weir GJ. Acquired hyperoxaluria with regional enteritis after ileal resection. Role of dietary oxalate. Ann Intern Med. 1973;79:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 211. | Nordenvall B, Backman L, Larsson L, Tiselius HG. Effects of calcium, aluminium, magnesium and cholestyramine on hyperoxaluria in patients with jejunoileal bypass. Acta Chir Scand. 1983;149:93-98. [PubMed] |
| 212. | Earnest DL, Gancher S, Admirand WH. Treatment of enteric hyperoxaluria with calcium and aluminium (abstract). Gastroenterology. 1976;70; A23/881. |
| 213. | Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 214. | Litvak DA, Iseki H, Evers BM, Greeley GH, Hellmich MR, Iwase K, Balasubramaniam A, Townsend CM. Characterization of two novel proabsorptive peptide YY analogs, BIM-43073D and BIM-43004C. Dig Dis Sci. 1999;44:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 215. | Walker-Smith JA, Phillips AD, Walford N, Gregory H, Fitzgerald JD, MacCullagh K, Wright NA. Intravenous epidermal growth factor/urogastrone increases small-intestinal cell proliferation in congenital microvillous atrophy. Lancet. 1985;2:1239-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 216. | Sullivan PB, Brueton MJ, Tabara ZB, Goodlad RA, Lee CY, Wright NA. Epidermal growth factor in necrotising enteritis. Lancet. 1991;338:53-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 217. | Rokkas T, Vaja S, Murphy GM, Dowling RH. Aminoguanidine blocks intestinal diamine oxidase (DAO) activity and enhances the intestinal adaptive response to resection in the rat. Digestion. 1990;46 Suppl 2:447-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 218. | Scolapio JS, Camilleri M, Fleming CR, Oenning LV, Burton DD, Sebo TJ, Batts KP, Kelly DG. Effect of growth hormone, glutamine, and diet on adaptation in short-bowel syndrome: a randomized, controlled study. Gastroenterology. 1997;113:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 187] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 219. | Szkudlarek J, Jeppesen PB, Mortensen PB. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: a randomised, double blind, crossover, placebo controlled study. Gut. 2000;47:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 220. | Campbell FC, Tait IS, Flint N, Evans GS. Transplantation of cultured small bowel enterocytes. Gut. 1993;34:1153-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 221. | Grant D. Current results of intestinal transplantation. The International Intestinal Transplant Registry. Lancet. 1996;347:1801-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 171] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 222. | Grant D. Intestinal transplantation: 1997 report of the international registry. Intestinal Transplant Registry. Transplantation. 1999;67:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 223. | Messing B, Lémann M, Landais P, Gouttebel MC, Gérard-Boncompain M, Saudin F, Vangossum A, Beau P, Guédon C, Barnoud D. Prognosis of patients with nonmalignant chronic intestinal failure receiving long-term home parenteral nutrition. Gastroenterology. 1995;108:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 224. | Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 392] [Article Influence: 15.1] [Reference Citation Analysis (0)] |