Published online Aug 15, 2001. doi: 10.3748/wjg.v7.i4.515
Revised: May 13, 2001
Accepted: May 20, 2001
Published online: August 15, 2001
AIM: To investigate the relationship between the expression of p16 gene and the gastric carcinogenesis, depth of invasion and lymph node metastases, and to evaluate the deletion and mutation of exon 2 in p16 gene in gastric carcinoma.
METHODS: The expression of P16 protein was examined by streptavidin-peroxidase conjugated method (S-P); the deletion and mutation of p16 gene were respectively examined by polymerase chain reaction (PCR) and polymerase chain reaction single strand conformation polymorphism analysis (PCR-SSCP) in gastric carcinoma.
RESULTS: Expression of P16 protein was detected in 96.25% (77/80) of the normal gastric mucosa, in 92.00% (45/50) of the dysplastic gastric mucosa and in 47.54% (58/122) of the gastric carcinoma. The positive rate of P16 protein expression in gastric carcinoma was significantly lower than that in normal gastric mucosa and dysplastic gastric mucosa (P < 0.05). The positive rate of P16 protein expression in mucoid carcinoma 10.00% (1/10) was significantly lower than that in poorly differentiated carcinoma 51.22% (21/41), undifferentiated carcinoma 57.69% (15/26) and signet ring cell carcinoma 62.50% (10/16) (P < 0.05). The positive rate of p16 protein in 30 cases paired primary and lymph node metastatic gastric carcinoma: There was 46.67% (14/30) in primary gastric carcinoma, 16.67% (5/30) in lymph node metastatic gastric carcinoma. The positive rate of lymph node metastatic carcinoma was significantly lower than that of primary carcinoma (P < 0.05). There was of p16 gene mutation in exon 2, but 5 cases displayed deletion of p16 gene in exon 2 in the 25 primary gastric carcinomas.
CONCLUSIONS: The expression loss of P16 protein related to the gastric carcinogenesis, gastric carcinoma histopathological subtypes and lymph merastasis. The mutation of p16 gene in exon 2 may not be involved in gastric carcinogenesis. But the deletion of p16 gene in exon 2 may be involved in gastric carcinogenesis.
-
Citation: He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deleton and mutation of
p16 gene in human gastric cancer. World J Gastroenterol 2001; 7(4): 515-521 - URL: https://www.wjgnet.com/1007-9327/full/v7/i4/515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i4.515
Carcinogenesis and progression of human gastric cancer are related to the activation of proto-oncogenes and/or the inactivation of anti-oncogenes and they are the results of genetic alteration accumulated. A recently cloned new tumor suppressor p16 gene is located in 9p21, with the full-length of 8.5 kb. It consists of 2 introns and 3 exons, encoding P16 protein-whose molecular mass is 15840 Mr, a single strain peptide comprising 148 amino acid, participating in regulating the proliferation of normal cell growth negatively[1,2]. There was a high frequent loss of homozygosis of p16 gene in a variety of cancer cell lines such as gliocytoma, melanoma, breast cancer cell lines[2] and in certain primary cancer, for example, leukemia[3], gliomas[4], astrocytomas[5], bladder cancer[6], melanoma[7], oral squamous cell carcinomas[8], squamous cell carcinoma of head and neck neoplasm[9,10]. The frequency of p16 gene deletion and mutation is up to 75% in all kinds of human neoplasm, higher than that of the well-known p53 gene. Gastric cancer is common in China[11-30]. In this paper, S-P immunohistochemical staining was used to detect the expression of P16 protein in gastric cancer and precancerous lesions. PCR and PCR-SSCP methods were used to analyse the deletion and mutation of p16 gene exon 2. This study aims to evaluate the relationship between P16 protein and the carcinogenesis, progression, histological types as well as biologic behaviors in human gastric cancer, to find a new marker in early diagnosis and to discover the role of deletion and mutation of p16 gene in exon 2 in the carcinogenesis and progression of human gastric cancer.
All specimens were confirmed by pathology. Paraffin-embedded tissue were collected from the department of pathology and fresh resected specimens were from the First Affiliated Hospital of the Nanhua University, among which there were 50 cases of dysplasia of gastric mucosa and 122 cases of gastric cancer (25 cases were resected freshly from September 1995 to December 1996). In the 122 cases of gastric cancer, 29 were well-differentiated adenocarcinoma, 41 were poorly-differentiated adenocarcinoma, 26 were undifferentiated carcinoma, 16 were signet ring cell carcinoma and the other 10 were mucoid carcinoma. There were 81 men and 41 women, 22 aged below 40 years, 69 aged from 41 to 59 years, and 31 were older than 60 years. The youngest was 15 years and the oldest 79 years ( mean 56 years). Superficial muscles, were invated in 50 cases and deep muscles and the full layer in 72. Sixty-nine cases had lymph node metastasis, 53 had no lymph node metastasis. Thirty cases primary and lymph node metastasis cancer selected randomly were paired and compared. According to Borrmann’s classification, 15 were type I, 43 were type II, 47 were type III and 17 were type IV. The 25 cases of fresh resected specimens included cancer, cancer-surroundings and normal mucosa selected far from cancer, were cut into 2-4 blocks under sterile conditions. Each block was 2-3 mm3 and stored in -70 °C refrigerator for PCR and PCR-SSCP analysis. The rest tissues were fixed in 100 mL·L-1 neutral formalin, resected, dehydrated, cleaned and paraffin-embedded. All paraffin-embedded tissues were cut into sequential slices for 5 µm and adhered to the glass which was processed by poly-lys previously.
Rabbit-anti-human P16 protein multiple clonal antibody, streptavidin-peroxidase immunozator kit (S-P kit), and DAB were all bought from Maxim Company, USA. Protase K (Merk, USA), Sma I, agar gel, propylene acrylamide, N-N-sulmethyl bipropylene acrylamide, ammonium persulfate, xylene nitrile, bromophenol blue were bought from Shanghai Sangon Company. PCR primer synthesized by Shanghai Sangon, primer sequences of p16 gene exon 2[4]. Sense: 5’-TCTGACCATTCTGTTCTCTC-3’ Antisense: 5’-CTCAGCTTTGGAAGCTCTCA-3’ The fragment length of amplification was 384 bp. Primer sequences of β-active served as an internal control. Sense: 5’-GCGGGGCGCCCCAGGCACCA-3’ antisense: 5’-CTCCTTAATGTCACGCAC GATTTC-3’ The fragment length of amplification was 548 bp.
Instrument Ultra low refrigerator (Japan) of -70 °C, rotary sector (Germany), microscope (Japan), type 480 DNA amplificatory (PE, USA), type 901 ultraviolet spectrophometer (PE, USA), type DY-IIIB vertical eletrophores and all kinds of centrifuges(Beijing Liuyi).
Operated as the specification of sp kit, that was: paraffin-embedded tissue slices deparaffined hydrated→endogenous peroxidase blocked→added first antibody→then bridge antibody→added enzyme labeled S-P reagents→DAB colorized→hematoxylin stained→dehydrated→cleaned and paraffin-embedded→observed by microscope.
Frozen tissue of 0.5 g was put into liquid nitrogen and powdered immediately, 10 × buffer (10 mmol·L-1 Tris-HCl pH8.0, 0.1 mol·L-1 EDTA pH8.0, 5 g·L-1 SDS) was added and span in 37 °Cwater for 1 h at the same time, added protase K to the mixture at a final concentration of 100 mg·L-1 in 50 °C water for 3 h and readjusted the protase K as possible reaction. After the mixture lysed completely, 20 mg·L-1 Rnase reacted in 37 °C water for 1 h, saturated phony was put together and bugged slightly for 10 min, centrifuged and extracted up clean liquid transfer to a cleaned plastic tube, saturated phony processed repeatedly 3 times, added 1/10 volume 3 mol·L-1 NaAc and 2-2.5 times cold ethyl, DNA precipitated by centrifugation, removed ethyl, DNA washed by 700 mL·L-1 ethyl land centrifuged 3 times, dried, resolved with TE, A260/A280: 1.8-1.9, stored at 0 °C for use.
PCR was performed according to the reference[31] in 50 µL reactive volume containing 0.1 µg gDNA template, 200 µmol·L-1 each of dCTP, DATP, dGTP, dTTP, 0.25 µmol·L-1 primer, PCR buffer (Tris-HCl 10 mmol·L-1, pH8.3, MgCl2 1.5 µmol·L-1, KCl 50 mmol·L-1, gelatin 100 mg·L-1) pre-denatured at 95 °C for 5 min and added 1.5 µL of Taq DNA polymerase, 75 µL of mineral oil. These samples were subjected to 30 cycles, including: 95 °C 1 min, 60 °C 1 min, 72 °C 1 min, and extended at 72 °C 5 min. Five µL of PCR product and appropriate bromophenol blue was added to the sample point container and electrophoresed at 20 g·L-1 agarose gel containing 0.5 mg·L-1 ethidium bromide at tank with 0.5 × TBE liquid of electrophoresis, then observed and photographed with ultraviolet radiography.
Five µL digested PCR product mixed with 5 µL denatured dissolution (950 mL·L-1 forman mide, 20 mmol·L-1 EDTA, 0.05% bromophenol blue, 0.5 g·L-1 xylene nitrile) denatured at 95 °C 5 min and colded on ice. Solution processed as above was added to the gel containing 80 g·L-1 polypropylene acrylamide, vertical electrophoresed at 100 V for 4 h and gel stained with silver: fixed in 100 mL·L-1 alcohol for 10 min→oxidized in 100 g·L-1 nitric acid for 3 min→drip washed for 1 min with double distilled water→stained in 12 mmol·L-1 silver nitric acid for 20 min→drip washed for 1 min with double distilled water→showed appropriate color in 0.028 mol anhydrous sodium carbonate and 0.19 mL·L-1 formalin→ended reducing response by 100 mL·L-1 glacial acetic acid→drip washed with double distilled water→analysis results and photographed. P16 protein expression of confirmed positively cervix carcinoma served as positive control. PBS substituted with first antibody served as negative control.
According to Gevadts’ standard modefied slightly[32,33], nuclear or plasma stained brown-yellow as positive, (-) indicated no cell stained positive or only plasma stained or the number of nuclear stained positive less than 1 cell, (+) indicated the cells stained weakly or the number of stained cells less than 25%, (++) indicated the cells stained moderately or the stained cells covering about 26%-50%, (+++) indicated cells stained strongly or the number of stained cell more than 50%. The number of nuclear stained positively more than 2 cells per high time sight was considered to be positive. No folding, and no edging-effect fields were chosen to calculate 100 cells per 5 sights and evaluate the average number of positive cells. Positive cells discerned by two researchers alone and decided on the disagreements together. No products of PCR amplification were loss of homozygosis of p16 gene, and abnormal traces found in PCR-SSCP were considered gene mutation.
Chiq-square test was used P value less than 0.05 was considered to be statistically significant.
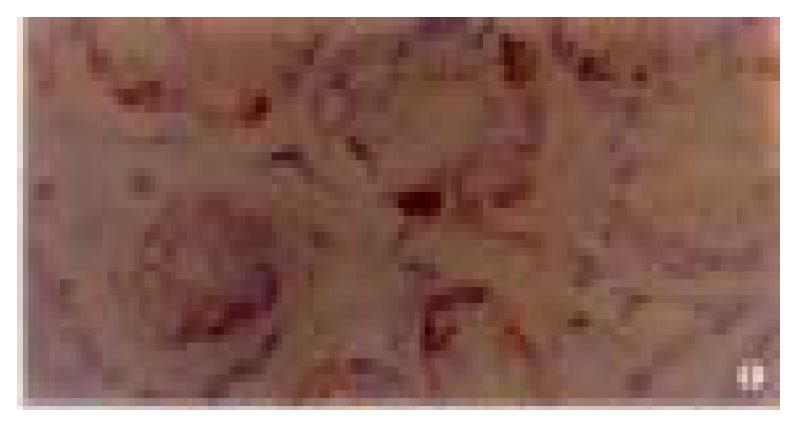
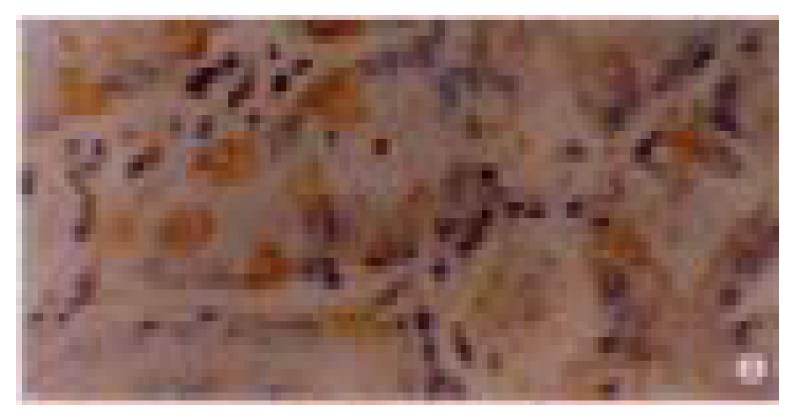
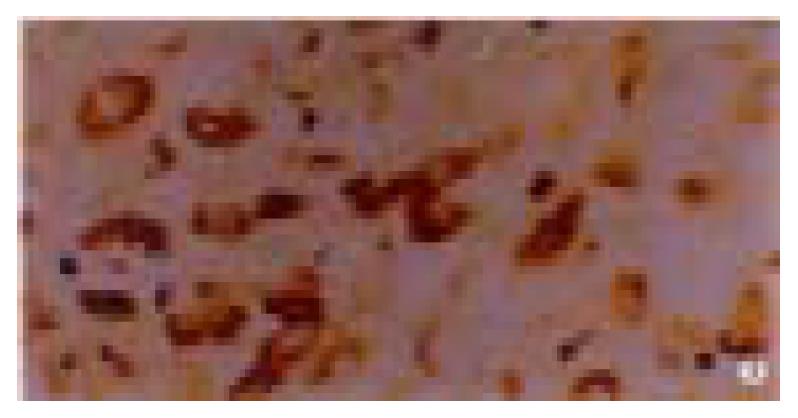
The positive rate of P16 protein expression in 80 cases of normal gastric mucosa was 96% (Figure 1), and in 50 cases of dysplasia mucosa was 90% (Figure 2). In these mucosa P16 protein expression could only be seen in partical adenoepithelial cells. We did not find staining in mucosal epithelial cells, matrix fibrocytes, lymphocytes and smooth myocytes. But in gastric cancer, the ratio was 48% (Figure 3). The positive rate of P16 protein expression in gastric cancer was lower than that in normal and dysplasia mucosa (P < 0.05). There was no significant difference between the normal gastric mucosa and dysplasia mucosa (P > 0.05, Table 1).
| Hisological types | n | P16 protein | p16 gene | |||||
| - | + | ++ | +++ | Positive rate (%) | Mutation | Deletion | ||
| A. Normal gastric mucosa | 80 | 3 | 41 | 20 | 16 | 96 | 0/25 | 0/25 |
| B. Dysplasia gastric mucosa | 50 | 5 | 12 | 19 | 14 | 92 | 0/25 | 0/25 |
| C. Gastric cancer | 122 | 64 | 13 | 20 | 25 | 48 | 0/25 | 5/25 |
In the 122 gastric cancer, the positive rate of P16 protein expression was 38%, 51%, 58%, 62% and 10% in well-differentiated adenocarcinoma, poorly-differentiated adenocarcinoma, undifferentiated carcinoma, signet ring cell carcinoma and mucoid carcinoma, respectively. The P16 protein expression in mucoid carcinoma was lower than that in signet ring cell carcinoma, undifferentiated carcinoma and poorly-differentiated adenocarcinoma (P < 0.05). The positive rate of P16 protein expression was 48% (24/50) in gastric cancer invaded superficial muscle layer and 47% (34/72) in gastric cancer invaded deep muscle and full layer. There was no apparent relevance between P16 protein expression and the depth of invasion (P > 0.05). In 30 cases of paired primary cancer and lymph node metastasis cancer, the rate of P16 protein expression of the lymph node metastasis cancer was 17% (5/30), significantly lower than that of primary cancer, 47% (14/30), (P < 0.05).
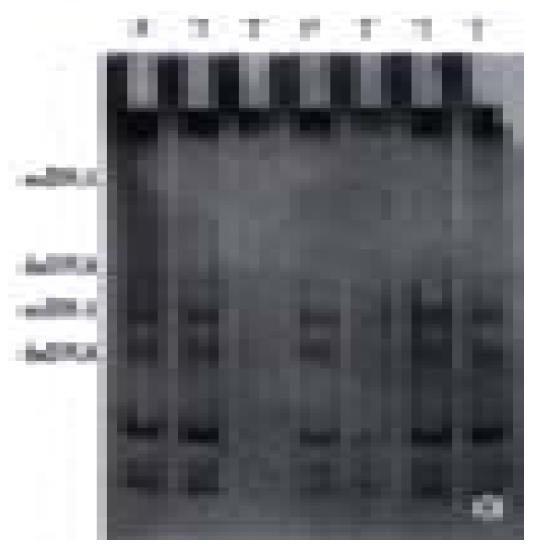
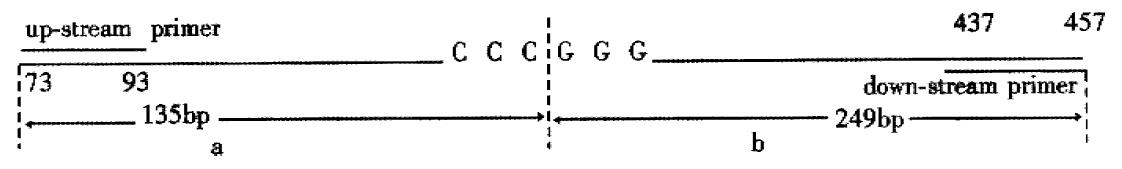
In 25 fresh resected gastric cancer, there were 7 well-differentiated adenocarcinoma, 13 poorly-differentiated adenocarcinoma, 3 undif ferentiated carcinoma, 1 signet ring cell carcinoma and 1 mucoid carcinoma, cancer-arrounding and normal mucosa were taken at the same time. The PCR amplification showed no product in 1 case of well-differentiated adenocarcinoma, 1 case of poorly-differentiated adenocarcinoma and 1 case of mucoid carcinoma; little product found in 1 case of well-differentiated adenocarcinoma and 1 case of poorly-differentiated adenocarcinoma. There were products of PCR amplification in the rest 20 cases of gastric cancer, tumor adjacent tissue and normal mucosa. All experiments were repeated three times. The result was identical. No product of PCR amplification might indicate the loss of homozygosis of p16 gene, little product of PCR amplification was possibly loss of heterozygosis of p16 gene or loss of homozygosis of p16 gene, but contaminated with normal mucosa (Figure 4). Four of these 5 cases were P16 protein negative expression and 1 case expressed weekly by immunohistochemical staining. No gene mutation was observed in PCR-SSCP analysis after the PCR amplification products cut with Sam I (Figure 5, Table 1) (the location of restriction site, and the length of fragment are shown in Figure 6).
The p16 gene is a tumor suppressor gene that participates in the negative regulation of the cell growth and proliferation, and a hot spot in the molecular biological research of neoplasm since its discovery in 1993. The product of p16 gene-P16 protein is the inhibitor of CDK4. Its function is to default the activity of CDK4 by binding with CDK4 against Cyclin D1 competitively, then inhibits the phosphation of Rb protein, transcription factor such as E2F when combined with the dephosphated Rb protein can not be released and activated, thus cells arrest in G0/G1 phase, resulting in cell dividing and proliferation suppressed. If p16 gene was abnormal, its function of negative regulation of cell growth would be lost. CDK4 combines to cyclin D1 and PRb phosphates, lots of transcription factors would be released. The cell from G1 phase enters into S phase rapidly, cell proliferates excessively and results in carcinogenesis and progression[1,2,34-36]. A lot of investigations show that there were P16 protein deletion and p16 gene abnormality in various primary cancers and cancer cell lines. The alteration forms of p16 gene were deletion, mutation[2,9,37-43], rearrangement, insertion[44-48], translocation[49-51]and hyper methylation of CpG islands presented in promotor sequence[52-58]. Such alterations consequently change the gene activity, cause abnormality, structure change of the product of p16 gene expression and the loss of its physiological functions. Recently, it has been reported that the exoteric p16 gene was transfected into the cancer cell which p16 gene deletes. The cancer cell restored p16 gene expression and cell growth was remarkably inhibited[59-67]. It has also been documented that the CpG island methylated of p16 gene cancer cell line was treated with 5-aza-2’-deoxycytidin. Cancer cell restored p16 gene expression and showed growth inhibition[68-71]. All these indicated that p16 gene and its product played important roles in the carcinogenesis.
This investigation showed that the positive rate of P16 protein expression in gastric cancer was remarkably lower than that in dysplasia and that in normal gastric mucosa (P < 0.05). The result indicated that gastric carcinogenesis was probably related to the loss of P16 protein expression. But there was no significant difference between the normal mucosa and the dysplasia mucosa of the stomach (P > 0.05). The positive rate of P16 protein expression in gastric cancer was not identical with other reports[72-73]. The cause was not clear. It was possibly related to the different standards of determination, reagents and some uncertain factors. However, the quantity of P16 protein expression increased from normal mucosa to precancerous lesions and gastric cancer (P < 0.05). Following pathological lesions, P16 protein expression increased. This change may inhibit cell proliferation. The positive rate of P16 protein expression in mucoid carcinoma was significantly lower than in poorly-differentiated adenocarcinoma, undifferentiated carcinoma and signet cell carcinoma (P < 0.05). The result suggested that the alteration of p16 gene was different in various histological types gastric cancer. The discrepancy of P16 protein expression exists in various histological types of lung and esophageal cancer[33,74]. There was difference of P16 protein expression and deletion of p16 gene in various differentiation types of gliomas. But the deletion of p16 gene concurred with the expression of P16 protein[4]. In 30 cases of primary gastric cancer paired with lymph node metastasis cancer, the positive expression rate of P16 protein in metastasis cancer was lower than in primary gastric cancer (P < 0.05), which was in agreement with the reported results[75]. The result convincingly suggested that the P16 protein deletion might be related to gastric cancer metastasis and indicate P16 protein expression heterogentity in gastric cancer[76]. What was more intriguing that 2 neighboring lymph nodes metastasis cancer migrated from primary cancer had positive expression P16 protein. Expression of P16 protein is not only related to neoplasms metastasis but also related to prognosis and progression. Expression of P16 protein is low, clinical prognosis is bad[77-81]. We also investigated the relevance between various factors such as age, sex, the depth of invasion and Borrman classification and P16 protein expression in gastric cancer. There was no significant difference (P > 0.05). The positive expression of P16 protein could merely be observed in partial adeno-epithelial cells of normal and dysplasia gastric mucosa, and weakly positive expression or undetectable in gastric mucosa epithelium cells, interstitial lymphocytes, fibroblasts and smooth muscle cells, which is contrary to some published files[82]. Nevertheless, others confer that the undetectability of P16 protein expression in neoplasm interstice[32], normal lung tissue[83] and normal urinoepithelial cells[82] might attribute to a paucity of P16 molecule in G0/G1[84] phase cells or short halftime of P16 protein[85].
Among some human neoplasms, p16 gene alterations always resided in exon 2[5]. There was no product of PCR amplification in 3 of 25 cases possibly due to the loss of homozygosis. Little product of PCR in 2 of 25 cases amplification might be the loss of heterozygosis or loss of homozygosis but normal mucosa contaminated. In the 5 cases, the expression of P16 protein was negative in 4 cases and weekly positive in one. The results manifested that 4 cases might be the loss of homozygosis and 1 case might be the loss of heterozygosis among the 5 cases of gastric cancer and the deletion of p16 gene is possibly related to the carcinogenesis and progression of gastric carcinoma. The rate of deletion in this study was slightly lower than that reported by others[74]. It was likely that only exon 2 was examined or inadequate for specimens or other unknown factors. Nevertheless, PCR amplification products were found in the rest 20 cases of gastric cancer, normal gastric mucosa and cancer-surrounding mucosa. No abnormal PAGE band and mutation of p16 gene was found by SSCP analysis digestion product of PCR amplification. We suggested that p16 gene was not involved in the carcinogenesis of gastric cancer, which coincided with other authors[86,87]. We also found that the frequency of p16 gene deletion was lower than that of deletion P16 protein expression. P16 protein was undetectable in normal and dysplasia gastric mucosa epithelial cells but in partial adenoepithelium. Some other uncertain mechanisms might exist in the regulation of p16 gene and the expression level of P16 protein[88-90], which require further studies.
Edited by Ma JY
| 1. | Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2515] [Cited by in RCA: 2557] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 2. | Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, Johnson BE, Skolnick MH. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 1888] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 3. | Faderl S, Kantarjian HM, Manshouri T, Chan CY, Pierce S, Hays KJ, Cortes J, Thomas D, Estrov Z, Albitar M. The prognostic significance of p16INK4a/p14ARF and p15INK4b deletions in adult acute lymphoblastic leukemia. Clin Cancer Res. 1999;5:1855-1861. [PubMed] |
| 4. | Nishikawa R, Furnari FB, Lin H, Arap W, Berger MS, Cavenee WK, Su Huang HJ. Loss of P16INK4 expression is frequent in high grade gliomas. Cancer Res. 1995;55:1941-1945. [PubMed] |
| 5. | Newcomb EW, Alonso M, Sung T, Miller DC. Incidence of p14ARF gene deletion in high-grade adult and pediatric astrocytomas. Hum Pathol. 2000;31:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Eissa S, Ali-Labib R, Khalifa A. Deletion of p16 and p15 genes In schistosomiasis-associated bladder cancer (SABC). Clin Chim Acta. 2000;300:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Piepkorn M. Melanoma genetics: an update with focus on the CDKN2A(p16)/ARF tumor suppressors. J Am Acad Dermatol. 2000;42:705-722; quiz 723-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Lin SC, Chang KW, Chang CS, Liu TY, Tzeng YS, Yang FS, Wong YK. Alterations of p16/MTS1 gene in oral squamous cell carcinomas from Taiwanese. J Oral Pathol Med. 2000;29:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Lang JC, Tobin EJ, Knobloch TJ, Schuller DE, Bartynski KJ, Mountain RE, Nicholson R, DeYoung BR, Weghorst CM. Frequent mutation of p16 in squamous cell carcinoma of the head and neck. Laryngoscope. 1998;108:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630-3633. [PubMed] |
| 11. | Tu SP, Jiang SH, Tan JH, Jiang XH, Qiao MM, Zhang YP, Wu YL, Wu YX. Proliferation inhibition and apoptosis induction by arsenic trioxide on gastric cancer cell SGC-7901. Shijie Huaren Xiaohua Zazhi. 1999;7:18-21. |
| 12. | Gao P, Jiang XW, Yuan WJ. Effects of gastrin and gastrin re-ceptor antagonist proglumide on gastric cancer line. Shijie Huaren Xiaohua Zazhi. 1999;7:22-24. |
| 13. | Li JQ, Wan YL, Cai WY. Biological significance of cyclin E expression in early gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:31-33. |
| 14. | Dong WG, Yu JP, Luo HS, Yu BP, Xu Y. Relationship between human papillomavirus infection and the development of gas-tric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:46-48. |
| 15. | Liu ZM, Shou NH. Expression significance of mdr1 gene in gastric carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 1999;7:145-146. |
| 16. | Shi YQ, Xiao B, Miao JY, Zhao YQ, You H, Fan DM. Construc-tion of eukaryotic expression vector pBK-fas and MDR rever-sal test of drug-resistant gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:309-312. |
| 17. | Fang DC, Zhou XD, Luo YH, Wang DX, Lu R, Yang SM, Liu WW. Microsatellite instability and loss of heterozygosity of suppressor gene in gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:479-481. |
| 18. | Yuan FP, Huang PS, Wang Y, Gong HS. Relationship between EBV infection in Fujian HCC and HBV and P53 protein expression. Shijie Huaren Xiaohua Zazhi. 1999;7:491-493. |
| 19. | Qin LJ. In situ hybridization of P53 tumor suppressor gene in human gastric precancerous lesions and gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:494-497. |
| 20. | Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its pre-cancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649-651. |
| 21. | Mi JQ, Zhang ZH, Shen MC. Significance of CD44v6 protein expression in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:156-158. |
| 22. | Gao GL, Yang Y, Yang S, Ren CW. Relationship between prolif-eration of vascular andothelial cells and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:282-284. |
| 23. | Wang RQ, Fang DC, Liu WW. MUC2 gene expression in gas-tric cancer and preneoplastic lesion tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:285-288. |
| 24. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax gene expression and its relationship with apoptosis in human gas-tric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. |
| 25. | Gu HP, Ni CR, Zhan RZ. Relationship between CD15 mRNA and its protein expression and gastric carcinoma invasion. Shijie Huaren Xiaohua Zazhi. 2000;8:851-854. |
| 26. | Wang DX, Fang DC, Liu WW. Study on alteration of multiple genes in intestinal metaplasia, atypical hyperplasia and gas-tric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:855-859. |
| 27. | Guo SY, Gu QL, Liu BY, Zhu ZG, Yin HR, Lin YZ. Experimental study on the treatment of gastric cancer by TK gene combined with mIL-2 gene. Shijie Huaren Xiaohua Zazhi. 2000;8:974-978. |
| 28. | Guo YQ, Zhu ZH, Li JF. Flow cytometric analysis of apoptosis and proliferation in gastric cancer and precancerous lesion. Shijie Huaren Xiaohua Zazhi. 2000;8:983-987. |
| 29. | Xia JZ, Zhu ZG, Liu BY, Yan M, Yin HR. Significance of immunohistochemically demonstrated micrometastases to lymph nodes in gastric carcinomas. Shijie Huaren Xiaohua Zazhi. 2000;8:1113-1116. |
| 30. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 31. | Lu SD. Chief editor.Current protocols for molecular biology, M No.1, Senior education press 1993. . |
| 32. | Geradts J, Hruban RH, Schutte M, Kern SE, Maynard R. Immunohistochemical p16INK4a analysis of archival tumors with deletion, hypermethylation, or mutation of the CDKN2/MTS1 gene. A comparison of four commercial antibodies. Appl Immunohistochem Mol Morphol. 2000;8:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Kratzke RA, Greatens TM, Rubins JB, Maddaus MA, Niewoehner DE, Niehans GA, Geradts J. Rb and p16INK4a expression in resected non-small cell lung tumors. Cancer Res. 1996;56:3415-3420. [PubMed] |
| 34. | Shapiro GI, Edwards CD, Rollins BJ. The physiology of p16(INK4A)-mediated G1 proliferative arrest. Cell Biochem Biophys. 2000;33:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol Cell Biol. 1998;18:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Ito Y, Takeda T, Sakon M, Monden M, Tsujimoto M, Matsuura N. Expression and clinical significance of the G1-S modulators in carcinoma of the extrahepatic bile duct. Anticancer Res. 2000;20:337-344. [PubMed] |
| 37. | Hashemi J, Platz A, Ueno T, Stierner U, Ringborg U, Hansson J. CDKN2A germ-line mutations in individuals with multiple cutaneous melanomas. Cancer Res. 2000;60:6864-6867. [PubMed] |
| 38. | Faderl S, Kantarjian HM, Estey E, Manshouri T, Chan CY, Rahman Elsaied A, Kornblau SM, Cortes J, Thomas DA, Pierce S. The prognostic significance of p16(INK4a)/p14(ARF) locus deletion and MDM-2 protein expression in adult acute myelogenous leukemia. Cancer. 2000;89:1976-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Cachia AR, Indsto JO, McLaren KM, Mann GJ, Arends MJ. CDKN2A mutation and deletion status in thin and thick primary melanoma. Clin Cancer Res. 2000;6:3511-3515. [PubMed] |
| 40. | Mochizuki S, Iwadate Y, Namba H, Yoshida Y, Yamaura A, Sakiyama S, Tagawa M. Homozygous deletion of the p16/MTS-1/CDKN2 gene in malignant gliomas is infrequent among Japanese patients. Int J Oncol. 1999;15:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Wang JC, Chen C. P16 gene deletions and point mutations in patients with agnogenic myeloid metaplasia (AMM). Leuk Res. 1999;23:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Orlow I, LaRue H, Osman I, Lacombe L, Moore L, Rabbani F, Meyer F, Fradet Y, Cordon-Cardo C. Deletions of the INK4A gene in superficial bladder tumors. Association with recurrence. Am J Pathol. 1999;155:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Kumar R, Smeds J, Lundh Rozell B, Hemminki K. Loss of heterozygosity at chromosome 9p21 (INK4-p14ARF locus): homozygous deletions and mutations in the p16 and p14ARF genes in sporadic primary melanomas. Melanoma Res. 1999;9:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Srivenugopal KS, Ali-Osman F. Deletions and rearrangements inactivate the p16INK4 gene in human glioma cells. Oncogene. 1996;12:2029-2034. [PubMed] |
| 45. | Nakamura M, Sugita K, Inukai T, Goi K, Iijima K, Tezuka T, Kojika S, Shiraishi K, Miyamoto N, Karakida N. p16/MTS1/INK4A gene is frequently inactivated by hypermethylation in childhood acute lymphoblastic leukemia with 11q23 translocation. Leukemia. 1999;13:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Orlow I, Drobnjak M, Zhang ZF, Lewis J, Woodruff JM, Brennan MF, Cordon-Cardo C. Alterations of INK4A and INK4B genes in adult soft tissue sarcomas: effect on survival. J Natl Cancer Inst. 1999;91:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | García-Sanz R, González M, Vargas M, Chillón MC, Balanzategui A, Barbón M, Flores MT, San Miguel JF. Deletions and rearrangements of cyclin-dependent kinase 4 inhibitor gene p16 are associated with poor prognosis in B cell non-Hodgkin's lymphomas. Leukemia. 1997;11:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Srivenugopal KS, Ali-Osman F. Deletions and rearrangements inactivate the p16INK4 gene in human glioma cells. Oncogene. 1996;12:2029-2034. [PubMed] |
| 49. | Duro D, Bernard O, Della Valle V, Leblanc T, Berger R, Larsen CJ. Inactivation of the P16INK4/MTS1 gene by a chromosome translocation t(9; 14)(p21-22; q11) in an acute lymphoblastic leukemia of B-cell type. Cancer Res. 1996;56:848-854. [PubMed] |
| 50. | Borg A, Sandberg T, Nilsson K, Johannsson O, Klinker M, Måsbäck A, Westerdahl J, Olsson H, Ingvar C. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst. 2000;92:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Monzon J, Liu L, Brill H, Goldstein AM, Tucker MA, From L, McLaughlin J, Hogg D, Lassam NJ. CDKN2A mutations in multiple primary melanomas. N Engl J Med. 1998;338:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 178] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Goussia AC, Agnantis NJ, Rao JS, Kyritsis AP. Cytogenetic and molecular abnormalities in astrocytic gliomas (Review). Oncol Rep. 2000;7:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Foster SA, Wong DJ, Barrett MT, Galloway DA. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol. 1998;18:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Kempster S, Phillips WA, Baindur-Hudson S, Thomas RJ, Dow C, Rockman SP. Methylation of exon 2 of p16 is associated with late stage oesophageal cancer. Cancer Lett. 2000;150:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Zhang J, Lai MD, Chen J. Methylation status of p16 gene in colorectal carcinoma and normal colonic mucosa. World J Gastroenterol. 1999;5:451-454. [PubMed] |
| 56. | Wong DJ, Barrett MT, Stöger R, Emond MJ, Reid BJ. p16INK4a promoter is hypermethylated at a high frequency in esophageal adenocarcinomas. Cancer Res. 1997;57:2619-2622. [PubMed] |
| 57. | Park SH, Jung KC, Ro JY, Kang GH, Khang SK. 5' CpG island methylation of p16 is associated with absence of p16 expression in glioblastomas. J Korean Med Sci. 2000;15:555-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Salem C, Liang G, Tsai YC, Coulter J, Knowles MA, Feng AC, Groshen S, Nichols PW, Jones PA. Progressive increases in de novo methylation of CpG islands in bladder cancer. Cancer Res. 2000;60:2473-2476. [PubMed] |
| 59. | Mobley SR, Liu TJ, Hudson JM, Clayman GL. In vitro growth suppression by adenoviral transduction of p21 and p16 in squamous cell carcinoma of the head and neck: a research model for combination gene therapy. Arch Otolaryngol Head Neck Surg. 1998;124:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Allay JA, Steiner MS, Zhang Y, Reed CP, Cockroft J, Lu Y. Adenovirus p16 gene therapy for prostate cancer. World J Urol. 2000;18:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Steiner MS, Zhang Y, Farooq F, Lerner J, Wang Y, Lu Y. Adenoviral vector containing wild-type p16 suppresses prostate cancer growth and prolongs survival by inducing cell senescence. Cancer Gene Ther. 2000;7:360-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Wolf JK, Kim TE, Fightmaster D, Bodurka D, Gershenson DM, Mills G, Wharton JT. Growth suppression of human ovarian cancer cell lines by the introduction of a p16 gene via a recombinant adenovirus. Gynecol Oncol. 1999;73:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Wang GL, Lo KW, Tsang KS, Chung NY, Tsang YS, Cheung ST, Lee JC, Huang DP. Inhibiting tumorigenic potential by restoration of p16 in nasopharyngeal carcinoma. Br J Cancer. 1999;81:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Sumitomo K, Shimizu E, Shinohara A, Yokota J, Sone S. Activation of RB tumor suppressor protein and growth suppression of small cell lung carcinoma cells by reintroduction of p16INK4A gene. Int J Oncol. 1999;14:1075-1080. [PubMed] |
| 65. | Lee KY, Yoo CG, Han SK, Shim YS, Kim YW. The effects of transferring tumor suppressor gene p16INK4A to p16INK4A-deleted cancer cells. Korean J Intern Med. 1999;14:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Miyakoshi J, Kitagawa K, Yamagishi N, Ohtsu S, Day RS, Takebe H. Increased radiosensitivity of p16 gene-deleted human glioma cells after transfection with wild-type p16 gene. Jpn J Cancer Res. 1997;88:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Allay JA, Steiner MS, Zhang Y, Reed CP, Cockroft J, Lu Y. Adenovirus p16 gene therapy for prostate cancer. World J Urol. 2000;18:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Loughran O, Malliri A, Owens D, Gallimore PH, Stanley MA, Ozanne B, Frame MC, Parkinson EK. Association of CDKN2A/p16INK4A with human head and neck keratinocyte replicative senescence: relationship of dysfunction to immortality and neoplasia. Oncogene. 1996;13:561-568. [PubMed] |
| 69. | Suh SI, Pyun HY, Cho JW, Baek WK, Park JB, Kwon T, Park JW, Suh MH, Carson DA. 5-Aza-2'-deoxycytidine leads to down-regulation of aberrant p16INK4A RNA transcripts and restores the functional retinoblastoma protein pathway in hepatocellular carcinoma cell lines. Cancer Lett. 2000;160:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Timmermann S, Hinds PW, Münger K. Re-expression of endogenous p16ink4a in oral squamous cell carcinoma lines by 5-aza-2'-deoxycytidine treatment induces a senescence-like state. Oncogene. 1998;17:3445-3453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2'-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95-101. [PubMed] |
| 72. | Zhao Y, Zhang XY, Shi XJ, Hu PZ, Zhang CS, Ma FC. Clinical significance of expressions of P16, P53 proteins and PCNA in gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:246-248. |
| 73. | Zhou Q, Zou JX, Chen YL, Yu HZ, Wang LD, Li YX, Guo HQ, Gao SS, Qiu SL. Alteration of tumor suppressor gene p16 and Rb in gastric cancinogesis. China Natl J New Gastroenterol. 1997;3:262. |
| 74. | Hayashi K, Metzger R, Salonga D, Danenberg K, Leichman LP, Fink U, Sendler A, Kelsen D, Schwartz GK, Groshen S. High frequency of simultaneous loss of p16 and p16beta gene expression in squamous cell carcinoma of the esophagus but not in adenocarcinoma of the esophagus or stomach. Oncogene. 1997;15:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Reed JA, Loganzo F, Shea CR, Walker GJ, Flores JF, Glendening JM, Bogdany JK, Shiel MJ, Haluska FG, Fountain JW. Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res. 1995;55:2713-2718. [PubMed] |
| 76. | He XS, Su Q, Qiang ZF, Lo ZY. Relation between expression of p16 protein and invasion and metastasis of gastric carcinoma. Aizheng. 2000;3:274,276. |
| 77. | Hui AM, Shi YZ, Li X, Takayama T, Makuuchi M. Loss of p16(INK4) protein, alone and together with loss of retinoblastoma protein, correlate with hepatocellular carcinoma progression. Cancer Lett. 2000;154:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Salvesen HB, Das S, Akslen LA. Loss of nuclear p16 protein expression is not associated with promoter methylation but defines a subgroup of aggressive endometrial carcinomas with poor prognosis. Clin Cancer Res. 2000;6:153-159. [PubMed] |
| 79. | Shiozaki H, Doki Y, Kawanishi K, Shamma A, Yano M, Inoue M, Monden M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery. 2000;127:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Straume O, Sviland L, Akslen LA. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res. 2000;6:1845-1853. [PubMed] |
| 81. | Myung N, Kim MR, Chung IP, Kim H, Jang JJ. Loss of p16 and p27 is associated with progression of human gastric cancer. Cancer Lett. 2000;153:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Reznikoff CA, Yeager TR, Belair CD, Savelieva E, Puthenveettil JA, Stadler WM. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 1996;56:2886-2890. [PubMed] |
| 83. | Shapiro GI, Edwards CD, Kobzik L, Godleski J, Richards W, Sugarbaker DJ, Rollins BJ. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 1995;55:505-509. [PubMed] |
| 84. | Tam SW, Shay JW, Pagano M. Differential expression and cell cycle regulation of the cyclin-dependent kinase 4 inhibitor p16Ink4. Cancer Res. 1994;54:5816-5820. [PubMed] |
| 85. | Shapiro GI, Park JE, Edwards CD, Mao L, Merlo A, Sidransky D, Ewen ME, Rollins BJ. Multiple mechanisms of p16INK4A inactivation in non-small cell lung cancer cell lines. Cancer Res. 1995;55:6200-6209. [PubMed] |
| 86. | Suh SI, Cho JW, Baek WK, Suh MH, Carson DA. Lack of mutation at p16INK4A gene but expression of aberrant p16INK4A RNA transcripts in human ovarian carcinoma. Cancer Lett. 2000;153:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Qin Y, Li B, Tan YS, Sun ZL, Zuo FQ, Sun ZF. Polymorphism of p16INK4a gene and rare mutation of p15INK4b gene exon2 in primary hepatocarcinoma. World J Gastroenterol. 2000;6:411-414. [PubMed] |
| 88. | Song SH, Jong HS, Choi HH, Kang SH, Ryu MH, Kim NK, Kim WH, Bang YJ. Methylation of specific CpG sites in the promoter region could significantly down-regulate p16(INK4a) expression in gastric adenocarcinoma. Int J Cancer. 2000;87:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 89. | Schneider BG, Gulley ML, Eagan P, Bravo JC, Mera R, Geradts J. Loss of p16/CDKN2A tumor suppressor protein in gastric adenocarcinoma is associated with Epstein-Barr virus and anatomic location in the body of the stomach. Hum Pathol. 2000;31:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Shim YH, Kang GH, Ro JY. Correlation of p16 hypermethylation with p16 protein loss in sporadic gastric carcinomas. Lab Invest. 2000;80:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |