Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.440
Revised: April 3, 2001
Accepted: April 12, 2001
Published online: June 15, 2001
- Citation: Wang LS, Zhu HM, Zhou DY, Wang YL, Zhang WD. Influence of whole peptidoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol 2001; 7(3): 440-443
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.440
Bifidobacteria are physiologically beneficial bacteria which are predominant in human intestine, and possess the most important functions. They play an important role in maintaining microbial balance of the intestine. Furthermore, their presence is thought to be an important indication of health of the body[1-4]. Whole peptidoglycan (WPG) is the major component in the cell wall of bifidobacterium, which is also a biological response modifier with nontoxic side effects. At present, many scholars have demonstrated that bifidobacteria can activate macrophages, T lymphocytes and natural killer cells to secrete many kinds of cytokines and important mediators[5-8]. In our report, the level of IL-6, IL-12 and TNF-α produced by macrophages is detected by employing laser scanning confocal microscopy, when these cells are stimulated by WPG of bifidobacteria bifidum. Simultaneously, the content of nitric oxide (NO) secreted from the macrophages is investigated by utilizing Griess reagent. Our goal is to explore the roles of these cytotoxic effector molecules on adjusting immune reaction of bifidobacterial WPG.
Whole peptidoglycan It was extracted from the cell wall of bifidobacteria bifidum according to the method described by Sekine and his colleagues, donated by Dr. Hu, who worked at department of examination of Zhongjing Medical University. Simultaneously, it had been evaluated[9].
Experimental animals BALB/c nude mice were purchased from the Experimental Animal Center of the First Military Medical University, 6-8 weeks in age, 18-22 g in weight, and housed in the SPF animal room.
Division of the experimental group and management of WPG The experimental animals were divided into two groups: the WPG injection group: 10 nude mice, 2 mL of 100 g·L-1 thioglycollate was injected into the nude mice intraperitoneally on d 1, 0.25 mg WPG (equivalent to 1 × 109 bifidobacteria) was injected intraperitoneally from d 2-6 continuously; and the control group: the number of animals and procedures were similar to the WPG injection group on d 1, 0.2 mL of PBS was injected intraperitoneally from d 2-6 continuously.
Collection and culture of peritoneal macrophages of nude mice All animals were killed on d 7. Abdomen skin of the nude mice was sterilized routinely. Cells were obtained by sterile lavage of the peritoneal cavity with cold D-hanks balanced salt solution. The lavage fluid was centrifuged at 1000 rpm for 10 min. The supernatant was decanted. The cells thus obtained were twice washed, resuspended in RPMI 1640 culture medium containing free fetal calf serum, then adjusted to be 1 × 109·L-1, and plated (2 × 105 cells/well) in 96-well tissue culture plates. Nonadherent cells were removed by repeated washing after 2 h incubation at 37 °C in 50 mL·L-1 CO2 in air. Adherent cells were macrophages. These cells were stimulated with 10 μg·L-1 LPS in RPMI 1640 containing 100 mL·L-1 FCS for 24 h. Supernatant was stored at -30 °C until assayed.
Detection of IL-6, IL-12 and TNF-α contents of peritoneal macrophages of nude mice Laser scanning confocal microscopy was utilized to detect the content of IL-6, IL-12 and TNF-α. The concrete protocol was as follows: ① The peritoneal cell suspension of nude mice was harvested and added to the modified Petri plate, and the desired peritoneal exudate cells were adjusted to 1 × 105 in 100 μL·plate-1. After incubated in a 50 mL·L-1 CO2 incubator at 37 °C for 2 h, these plates were washed vigorously to eliminate nonadherent cells, and macrophages were obtained. Then 500 μL of RPMI 1640 medium containing 10 μg·L-1 LPS was added to each plate. After 24 h, the supernatants were discarded, and the macrophages were washed with phosphate buffered saline (PBS) 3 times for 5 min; ② 100 g·L-1 non-specific bovine serum album was added to the plates which were incubated for 10 min at room temperature; ③ The plates were rinsed with PBS 3 times for 5 min; ④ 100 μL of rabbit anti-mouse IL-6, IL-12 and TNF-α monoclonal antibody (Genda Technology Corp. Canada) was added to the plates which were incubated for 60 min at 37 °C respectively; ⑤ The plates were rinsed with PBS 3 times for 5 min; ⑥ 100 μL of FITC labeled goat anti-rabbit IgG (Santa Cruz) was added to the plates which were incubated for 30 min at 37 °C. The antibody was used at a dilution of 1 in 100. ⑦ The plates were rinsed with PBS 3 times for 5 min; ⑧ The fluorescent intensity of macrophages was detected by employing laser scanning confocal microscopy with an arousement wavelength of 488 nm and a radiation wavelength of 475 nm. For each plate, average fluorescent value from beyond 100 macrophages in different fields was investigated, which was chosen to be quantitative parameter.
Griess reagent Naphthylethylenediamine hydrochloride (0.1%) (Sigma) was prepared with distilled water, and 1% sulfanilamide (Sigma) was prepared with 5% H3PO4. The two reagents were mixed equally before utilization.
Detection of the level of NO After 1 mmol·L-1 sodium nitrite (NaNO2) was diluted by means of doubling series, it was mixed with equal Griess reagent, then shaken for 10 min lightly at room temperature. At last, absorbance value (A) of triplicate samples was read on Bio-Rad 550 type microelisa reader using a test wavelength of 550 nm. One hundred microliter of each supernatant which was to be determining was mixed with 5 μL of 300 g·L-1 ZnSO4 to remove protein, then centrifuged at 5000 rpm for 10 min. The supernatant was collected, and mixed with equal Griess reagent, then shaken lightly for 10 minutes at room temperature. Absorbance value (A) was read on Bio Rad 550 type Microelisa Reader using a test wavelength of 550 nm.
The statistical analysis between WPG injection group and control group was made by means of Student's t test.
A standard curve was made by analyzing the relationship between different concentrations of standard NaNO2 and the corresponding A value assayed. Simultaneously, linear correlation and regression analysis was performed, and the linear regression equation was as follows: Y = 0.013 + 0.022X, r = 0.999. It suggested that there existed a fine correlation between the content of standard NaNO2 and the corresponding A value. The content of NO was obtained correspondingly when the A value of different samples substituted the linear regression equation. By means of the statistical analysis, we found that the content of NO produced by peritoneal macrophages of nude mice in WPG injection group was significantly higher than in the control group (P < 0.01, Table 1).
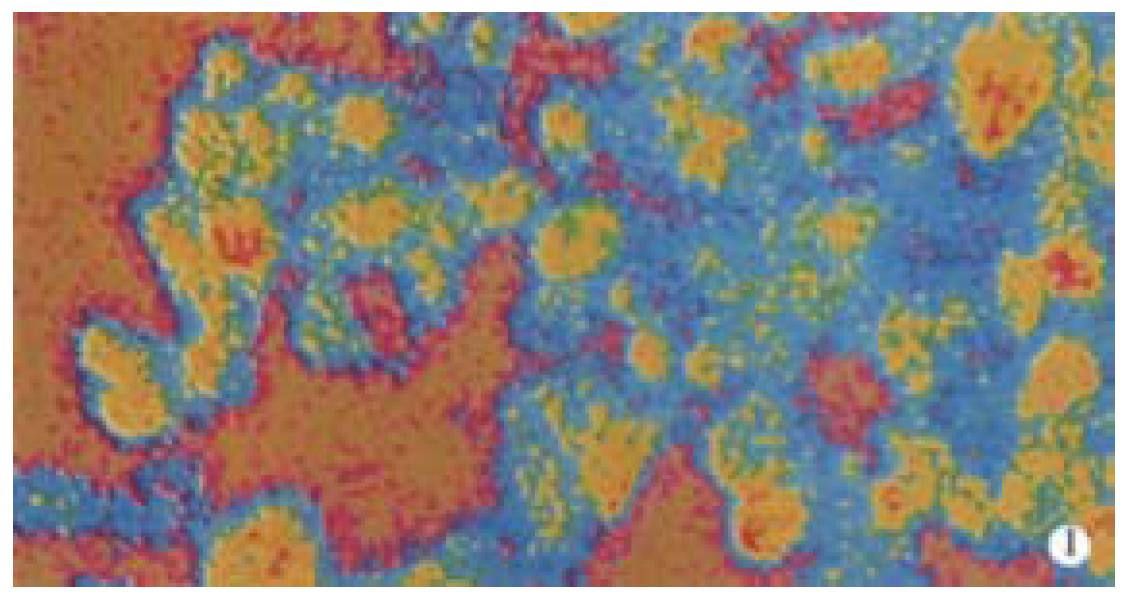
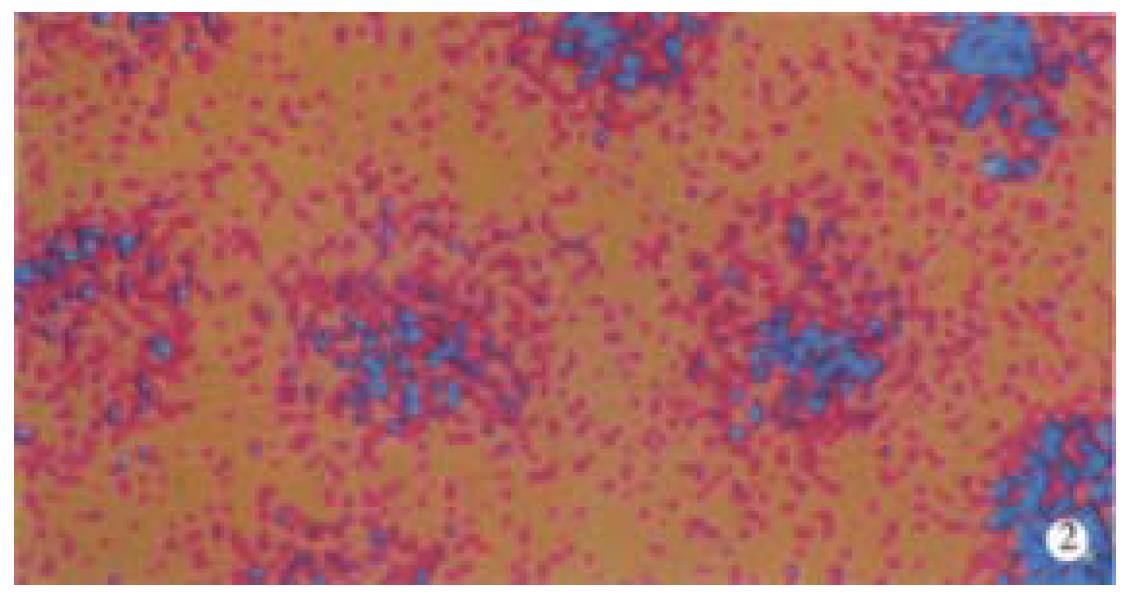
The content of IL-6, IL-12 and TNF-α produced by peritoneal macrophages of nude mice was detected by employing laser scanning confocal microscopy, when WPG of bifidobacteria bifidum was injected into nude mice intraperitoneally. The results suggested that the macrophages present different color. The macrophages in WPG injection group appeared white, red, yellow and blue which was narrow in area relatively, while, the macrophages in control group were mainly blue and less yellow. Different color reflected different fluorescent intensities. Analyzing with the ACAS software of laser scanning confocal microscopy, showed that the fluorescent intensity, which reflected the content of IL-6, IL-12 and TNF-α emitting from peritoneal macrophages of nude mice in WPG injection group, was markedly higher that in control group (P < 0.01, Table 1 and Figure 1, Figure 2).
WPG of bifidobacteria bifidum is a bag-form structure which consists of polysaccharides and peptidoglycans. It preserved the integral structure of whole cells, and possessed some important biological characteristics, such as relaxation of senescence, antitumor, control of infection, antimutation, etc[10-14]. Furthermore, it could also activate macrophages of immune system of the body. Tejada-Simon and his colleagues demonstrated that WPG of bifidobacteria infantis could activate RAW 264.7 macrophage cell line to produce a lot of TNF-α and NO in vitro[8]. In our report, the fluorescence intensity of IL-1, IL-6 and TNF-α and the content of NO derived from the peritoneal macrophages of nude mice was significantly elevated when WPG of bifidobacteria bifidum was injected into nude mice intraperitoneally. It was indicated that WPG of bifidobacteria bifidum could activate macrophages to secrete a large amount of cytotoxic effector molecules.
IL-1, IL-6 and TNF-α was the important cytokines produced by activated macrophages. They could act many aspects of immune system. IL-6 could promote the differentiation and maturation of B lymphocytes, and stimulate these cells to secrete antibodies. Furthermore, it also could induce proliferation and activation of resting T lymphocytes directly[15,16]. IL-12 could induce the production of IFN-γ by resting and activated T lymphocytes and natural killer (NK) cells, and possess the ability to act as a LAK cell growth factor[17-20]. TNF-α could augment the antitumor ability of NK, CTL and LAK cells, and play an important role in adjusting the activation of T lymphocytes[21,22]. These cytokines had broard antitumor and antimetastatic activities in vivo and in vitro markedly[23-35]. NO was the signal molecules and effector molecules which had broard biological activities. It was also the important effector molecules that activated macrophages killing tumor cells and pathogenic micro-organisms[36-41]. The induction of these cytotoxic effector molecules may play an important role in antitumor immune reaction of WPG. It was widely acknowledged that WPG could obviously inhibit the growth of many kinds of tumors in vivo. Rhee and his colleagues demonstrated that WPG of bifidobacteria spp. Exhibited markedly antitumor activity against subcutaneously transplanted sarcoma 180 in mice[42]. Ishihara, et al[43] reported that the volume of metastatic skin melanoma obviously decreased, when intralesional administration of WPG of bifidobacteria infantis was prepared. We had also found that WPG of bifidobacterium bifidum could induce apoptosis of the colorectal carcinoma transplantation neoplasms of nude mice, and inhibit its proliferation simultaneously. In our report, WPG could activate macrophages to secrete a lot of IL-6, IL-12, TNF-α and NO. Because these important mediators present obvious antitumor activity, the cytotoxic effector molecules produced by activated macrophages may mediate the effect on antitumor of WPG.
Li-Sheng Wang, graduated and obtained PhD, from the First Military Medical University in 1998, now working at Department of Gastroenterology, Shenzhen Municipal People's Hospital, Jinan University of Medical Sciences, having 35 papers published.
Edited by Ma JY
| 1. | Kopp-Hoolihan L. Prophylactic and therapeutic uses of probiotics: a review. J Am Diet Assoc. 2001;101:229-238; quiz 239-241. [PubMed] |
| 2. | Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 414] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | de Roos NM, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr. 2000;71:405-411. [PubMed] |
| 4. | Del Re B, Sgorbati B, Miglioli M, Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol. 2000;31:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 443] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | von Wright A, Salminen S. Probiotics: established effects and open questions. Eur J Gastroenterol Hepatol. 1999;11:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Yasui H, Shida K, Matsuzaki T, Yokokura T. Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71:1682S-167S; discussion 1682S-167S;. [PubMed] |
| 8. | Tejada-Simon MV, Pestka JJ. Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J Food Prot. 1999;62:1435-1444. [PubMed] |
| 9. | Le J, Hu H. Isolation and purification of whole peptidoglycan from bifidobacterium. Zhongguo Weishengtaixue Zazhi. 1997;9:10-13. |
| 10. | Wang LS, Pan LJ, Shi L, Zhang YL, Zhou DY. The inhibition function of Bifidobacteria to experimental large bowel carcinoma and the primary exploration of its mechanisms. Huaren Xiaohua Zazhi. 1998;6:456-457. |
| 11. | Wang LS, Pan LJ, Li MS, Sun Y, Shi L, Zhang YL, Zhou DY. The apoptosis of experimental large bowel carcinoma induced by whole peptidoglycan of Bifidobacteria by using in situ end labeling method. Shijie Huaren Xiaohua Zazhi. 1999;7:710. |
| 12. | Wang LS, Pan LJ, Chen CL, Li MS, Shun Y, Zhang YL, Zhou DY. Effect of Bifidobacterium on proliferation and apoptosis of experimental large bowel carcinoma in situ. Shijie Huaren Xiaohua Zazhi. 2000;8:429-431. |
| 13. | Horie H, Kanazawa K, Okada M, Narushima S, Itoh K, Terada A. Effects of intestinal bacteria on the development of colonic neoplasm: an experimental study. Eur J Cancer Prev. 1999;8:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Gallaher DD, Khil J. The effect of synbiotics on colon carcinogenesis in rats. J Nutr. 1999;129:1483S-1487S. [PubMed] |
| 15. | Worth LL, Jia SF, An T, Kleinerman ES. ImmTher, a lipophilic disaccharide derivative of muramyl dipeptide, up-regulates specific monocyte cytokine genes and activates monocyte-mediated tumoricidal activity. Cancer Immunol Immunother. 1999;48:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Xiang J, Moyana T. Regression of engineered tumor cells secreting cytokines is related to a shift in host cytokine profile from type 2 to type 1. J Interferon Cytokine Res. 2000;20:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bennett IM, Zatsepina O, Zamai L, Azzoni L, Mikheeva T, Perussia B. Definition of a natural killer NKR-P1A+/CD56-/CD16- functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med. 1996;184:1845-1856. [PubMed] |
| 18. | Nakui M, Ohta A, Sekimoto M, Sato M, Iwakabe K, Yahata T, Kitamura H, Koda T, Kawano T, Makuuchi H. Potentiation of antitumor effect of NKT cell ligand, alpha-galactosylceramide by combination with IL-12 on lung metastasis of malignant melanoma cells. Clin Exp Metastasis. 2000;18:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Chen Y, Emtage P, Zhu Q, Foley R, Muller W, Hitt M, Gauldie J, Wan Y. Induction of ErbB-2/neu-specific protective and therapeutic antitumor immunity using genetically modified dendritic cells: enhanced efficacy by cotransduction of gene encoding IL-12. Gene Ther. 2001;8:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Okamoto M, Gohda H, Ohe G, Yoshida H, Matsuno T, Saito M, Sato M. Cytokine-inducing activity and antitumor effect of a liposome-incorporated interferon-gamma-inducing molecule derived from OK-432, a streptococcal preparation. J Immunother. 2000;23:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Li DH, Havell EA, Brown CL, Cullen JM. Woodchuck lymphotoxin-alpha, -beta and tumor necrosis factor genes: structure, characterization and biological activity. Gene. 2000;242:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Champelovier P, Richard MJ, Seigneurin D. Autocrine regulation of TPA-induced apoptosis in monoblastic cell-line U-937: role for TNF-alpha, MnSOD and IL-6. Anticancer Res. 2000;20:451-458. [PubMed] |
| 23. | Cao X, Wang Q, Ju DW, Tao Q, Wang J. Efficient inducation of local and systemic antitumor immune response by liposome-mediated intratumoral co-transfer of interleukin-2 gene and interleukin-6 gene. J Exp Clin Cancer Res. 1999;18:191-200. [PubMed] |
| 24. | Kang HS, Cho DH, Kim SS, Pyun KH, Choi I. Antitumor effects of IL-6 on murine liver tumor cells in vivo. J Biomed Sci. 1999;6:142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Rancourt C, Piché A, Gomez-Navarro J, Wang M, Alvarez RD, Siegal GP, Fuller GM, Jones SA, Curiel DT. Interleukin-6 modulated conditionally replicative adenovirus as an antitumor/cytotoxic agent for cancer therapy. Clin Cancer Res. 1999;5:43-50. [PubMed] |
| 26. | Lasek W, Mackiewicz A, Czajka A, Switaj T, Goł b J, Wiznerowicz M, Korczak-Kowalska G, Bakowiec-Iskra EZ, Gryska K, Izycki D. Antitumor effects of the combination therapy with TNF-alpha gene-modified tumor cells and interleukin 12 in a melanoma model in mice. Cancer Gene Ther. 2000;7:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Lissoni P. Modulation of anticancer cytokines IL-2 and IL-12 by melatonin and the other pineal indoles 5-methoxytryptamine and 5-methoxytryptophol in the treatment of human neoplasms. Ann N Y Acad Sci. 2000;917:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Gajewski TF, Fallarino F, Ashikari A, Sherman M. Immunization of HLA-A2+ melanoma patients with MAGE-3 or MelanA peptide-pulsed autologous peripheral blood mononuclear cells plus recombinant human interleukin 12. Clin Cancer Res. 2001;7:895s-901s. [PubMed] |
| 29. | Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Endogenous IL-12 is necessary for rejection of P815 tumor variants in vivo. J Immunol. 1996;156:1095-1100. [PubMed] |
| 30. | Watanabe M, McCormick KL, Volker K, Ortaldo JR, Wigginton JM, Brunda MJ, Wiltrout RH, Fogler WE. Regulation of local host-mediated anti-tumor mechanisms by cytokines: direct and indirect effects on leukocyte recruitment and angiogenesis. Am J Pathol. 1997;150:1869-1880. [PubMed] |
| 31. | Golab J, Stoklosa T, Czajka A, Dabrowska A, Jakobisiak M, Zagozdzon R, Wojcik C, Marczak M, Wilk S. Synergistic antitumor effects of a selective proteasome inhibitor and TNF in mice. Anticancer Res. 2000;20:1717-1721. [PubMed] |
| 32. | Kuroda K, Miyata K, Tsutsumi Y, Tsunoda S, Nishimura K, Mitsuishi Y, Nakagawa S, Mayumi T. Preferential activity of wild-type and mutant tumor necrosis factor-alpha against tumor-derived endothelial-like cells. Jpn J Cancer Res. 2000;91:59-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Chambaut-Guérin AM, Costa SL, Lefrançois T, Fages C, Gauthereau X, Tardy M. Effects of retinoic acid and tumor necrosis factor alpha on GL-15 glioblastoma cells. Neuroreport. 2000;11:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Baher AG, Andres ML, Folz-Holbeck J, Cao JD, Gridley DS. A model using radiation and plasmid-mediated tumor necrosis factor-alpha gene therapy for treatment of glioblastomas. Anticancer Res. 1999;19:2917-2924. [PubMed] |
| 35. | Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999;59:6153-6158. [PubMed] |
| 36. | Ziche M, Morbidelli L. Nitric oxide and angiogenesis. J Neurooncol. 2000;50:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3127] [Cited by in RCA: 3071] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 38. | Bauer G. Reactive oxygen and nitrogen species: efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115-4139. [PubMed] |
| 39. | Wang B, Xiong Q, Shi Q, Le X, Abbruzzese JL, Xie K. Intact nitric oxide synthase II gene is required for interferon-beta-mediated suppression of growth and metastasis of pancreatic adenocarcinoma. Cancer Res. 2001;61:71-75. [PubMed] |
| 40. | Bruns CJ, Shinohara H, Harbison MT, Davis DW, Nelkin G, Killion JJ, McConkey DJ, Dong Z, Fidler IJ. Therapy of human pancreatic carcinoma implants by irinotecan and the oral immunomodulator JBT 3002 is associated with enhanced expression of inducible nitric oxide synthase in tumor-infiltrating macrophages. Cancer Res. 2000;60:2-7. [PubMed] |
| 41. | Farias-Eisner R, Chaudhuri G, Aeberhard E, Fukuto JM. The chemistry and tumoricidal activity of nitric oxide/hydrogen peroxide and the implications to cell resistance/susceptibility. J Biol Chem. 1996;271:6144-6151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Rhee YK, Bae EA, Kim SY, Han MJ, Choi EC, Kim DH. Antitumor activity of Bifidobacterium spp. isolated from a healthy Korean. Arch Pharm Res. 2000;23:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Wang LS, Pan LJ, Shi L, Li MS, Shun Y, Zhang YL, Zhou DY. The inhibition of whole peptidoglycan of bifidobacterium in experimental colorectal carcinoma and induction of apoptosis. Zhonghua Xiaohua Zazhi. 1999;19:331-334. |