Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.422
Revised: March 3, 2001
Accepted: April 12, 2001
Published online: June 15, 2001
- Citation: Yan FH, Zhou KR, Jiang YP, Shi WB. Inflammatory pseudotumor of the liver: 13 cases of MRI findings. World J Gastroenterol 2001; 7(3): 422-424
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.422
Inflammatory pseudotumor of the liver, a rare benign lesion, is often confused with the malignant tumors. Until 1998, less than 80 cases had been reported in the world[1,2]. The accuracy of the pre-surgical diagnosis was low[3,4], and very few materials on the diagnosis using dynamic MRI were reported[5-7]. We collected thirteen cases of inflammatory pseudotumor of the liver were collected and proved by pathology with MRI studies. Our purpose is to describe and analyze the MRI findings of this lesion and find out the valuable signs suggesting the diagnosis so as to avoid unnecessary operations.
Thirteen cases collected from September 1995 to December 2000 in our hospital (9 men and 4 women aged from 31-58 years, 41 years in average). More than half of the cases had no symptoms, and the others complained of upper abdominal discomfort, only three cases had fever of unknown causes. AFP and other liver lab testing were normal.
All of 13 cases underwent MR examinations with 1.5T Signa scanner (GE Medical Systems Milwaukee, WI), including T1WI (TR/TE = 500-700 ms/14-16 ms), T2WI SE sequence (TR/TE = 2000-4000 ms/60-90 ms) and FMPSPGR dynamic enhanced MRI(Matrix 256 × 128, thickness 7 mm, gap 3 mm, TR/TE Flip Angle = 100-150 ms/1.5-4.5 ms/60-90°). Four repeated acquisitions were obtained at 25-30, 60, 90 and 180 s respectively following power injection of 15-20 mL (0.15 mmol·kg-1) of Gd-DTPA (gadopentetic dimeglumine, Magnevist, Shering Pharmaceutical Ltd.) via an antecubital vein.
The MRI imagings of all cases were read and analyzed by two experienced doctors. Comparisons were made between preoperative diagnosis and results of surgical pathology.
A total of 16 lesions were identified by surgical pathology in the 13 cases, including three lesions in 1 case and single one in 12 cases. The size of the lesions was 0.8-3 cm in diameter (2.1 cm in average). The shape of the lesions was also variable, being round, ovoid, lobular (seen in coalescence of small focus) and irregular.
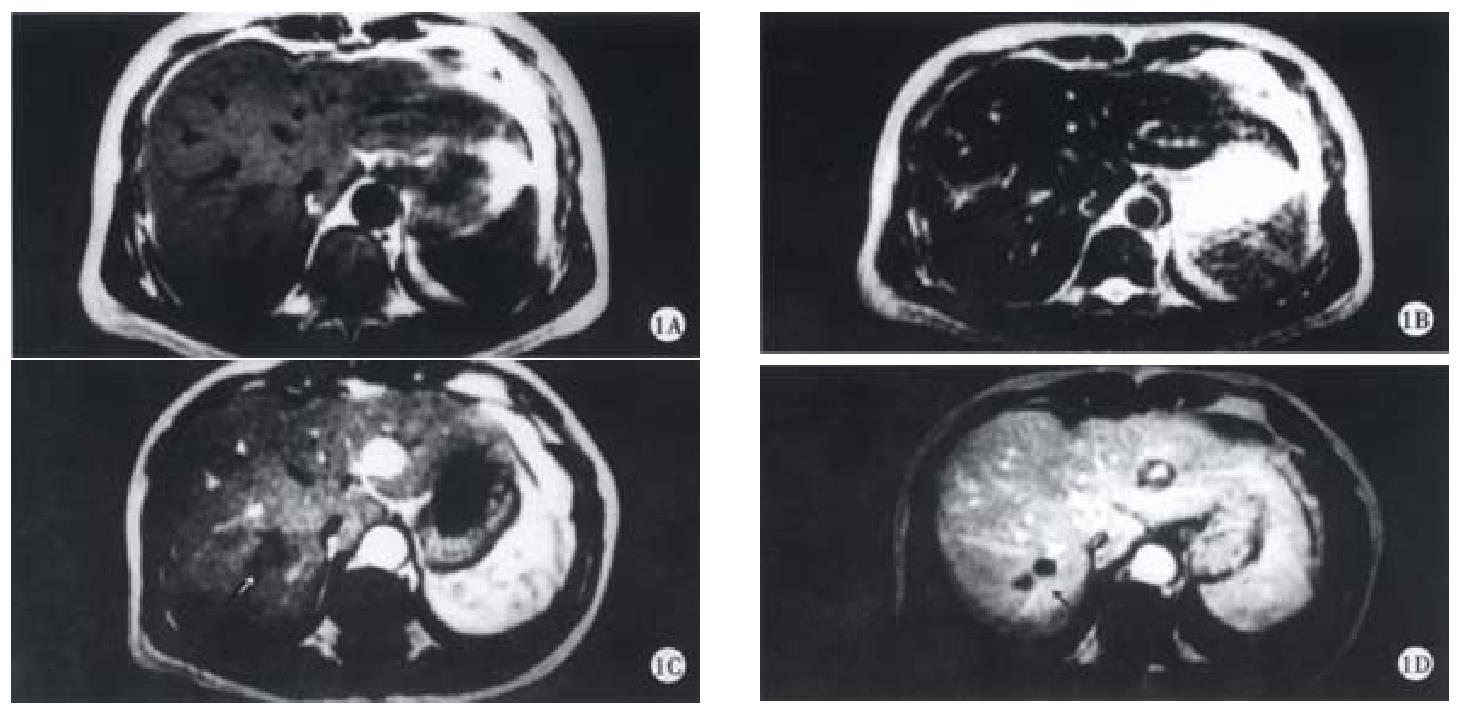
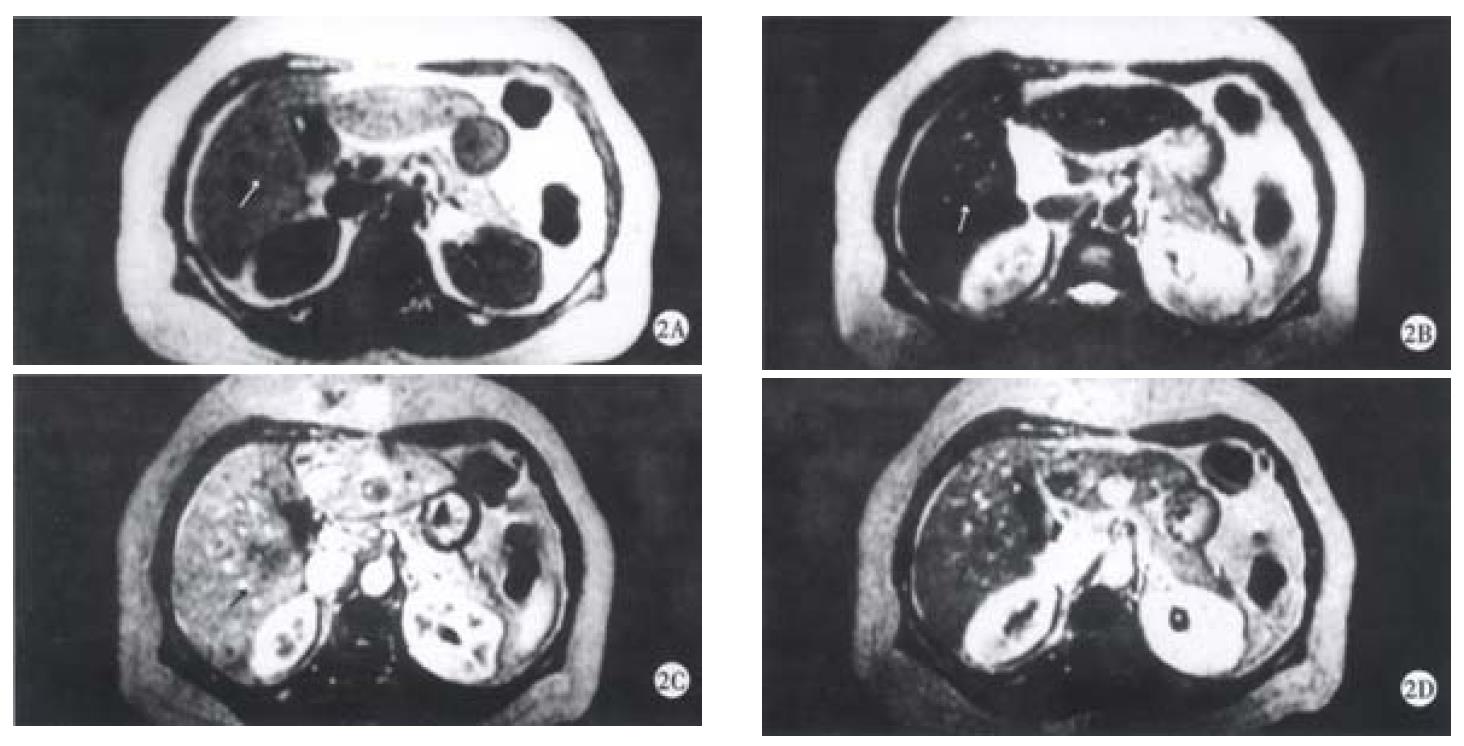
On SE sequence T1WI, 6 of 16 lesions were isointense (Figure 1A), the other 10 lesions were slightly hypointense (Figure 2A). On SE sequence T2WI, 6 of 16 lesions were slightly hyperintense (Figure 2B), the other 10 lesions were nearly equal intensity of signal to that of normal parenchyma (Figure 1B). The lesions discovered on SE sequence MRI were not well defined and the conspicuity between the lesions and the parenchyma were much less than that seen on dynamic MRI imaging of portal venous and delayed phases.
On dynamic enhanced MRI, none of the lesions showed enhancement in the dominant arterial phase (Figure 1C, Figure 2C). Therefore, the lesions were not seen clearly in this phase. On the contrary, all the lesions were well defined in the portal venous phase or/and the delayed phase. In these phases, the following appearances could be found: ① peripheral enhancement of the lesion (12/16, 75%) (Figure 2D). ② Small nodular enhancement (6/16, 37.50%), located in the central area of the lesions like core (Figure 2D), or near the marginal area of the lesions which we described as stalactite. ③ Septum within the lesions which could be linear or thick and irregular in appearance were seen in 8 (50%) of 16 lesions, and enhanced in the portal venous phase or/and delayed phase (Figure 1D). ④ All findings on dynamic MR imaging were seen overlapped.
Inflammatory pseudotumor of the liver is a rare benign disease, occasionally discovered by US, CT and MRI[5-9]. The etiology has not yet been very clear, and may be related to infection and immunogical compromise. Fibroblastic proliferation and chronic inflammatory cell infiltration constitute the characteristic features of the disease[3,10]. The mass is surrounded by the fibrocollageous stroma rich with capillary vessels, which may explain the peripheral enhancement in portal and delayed phases scans in the majority of cases due to the extravascular accumulation of contrast medium. Within the mass, there are less inflammatory cell components and more coagulative necrosis, so on SE T2WI, most of the lesions were nearly equal intensity of signal to that of normal parenchyma because of coagulative necrosis containing less free water. These are the characteristics of inflammatory pseudotumor of the liver. Six lesions showed slightly hyperintense on SE T2WI, because of more inflammatory cell infiltration within the lesions. The larger area consisted of coagulative necrosis and cell components in the central region does not enhance as fibrosis in later phase and appears heterogeneous. It was not enhanced in early arterial phase since the lesion had no direct hepatic arterial blood supply.
Most of the lesions could be detected by sonography in our group, but as it was hard to be characterized, color Doppler ultrasound became mandatory supplementary modality, which could recognize the presence or absence of arterial blood flow to help determine the nature of the lesions.
SE sequence of MRI had more limitations in detection of the lesions since the difference of signal intensity was not conspicuous between the lesion and parenchyma on T1WI as well as on T2WI. Six of 16 lesions in this group were isointense on T1WI and 10 of 16 lesions were also isointense on T2WI, and the others had only slight or moderate difference with the normal hepatic parenchyma. So, contrast enhanced MRI was very important to avoid potentially missing detection of small lesions and it is very important to recognize the nature of the lesion and not to confuse with the malignant tumors, mainly hepatocellular carcinoma and metastases of the liver.
The key point to differentiate it from hypervascular HCC is the absence of enhancement in arterial phase. Attention should be paid to the differentiation from hypovascular HCC. The following findings on MRI might be helpful: ① peripheral enhancement of lesions on the later phase; ② the presentation of septum and core, which has never been seen in HCC; ③ variable or irregular shape of the lesions, coalescence of several small lesions in one case; ④ most of the lesions were isointense on T2WI, but more than 90% lesions of HCC were hyperintense on T2WI[11]. MRI with mangafodipir trisodium might help distinguish inflammatory pseudotumor of the liver from hepatocellular carcinoma[12]. But peripheral ring-like enhancement may mimic the metastatic lesions. The history of primary malignant tumors and the absence of fibrotic septum or linear appearance within the mass and the most of lesions were hyperintense on T2WI could help the differentiation.
In summary, in view of rare occurrence, few cases of inflammatory pseudotumor of the liver have been accurately diagnosed preoperatively in the past. Since MRI was put into clinical application and with experiences accumulated, the accuracy of diagnosing this disease has been greatly improved.
In conclusion, MRI is very imperative to reveal the features of the lesions, such as no early arterial enhancement, peripheral enhancement, septum and small nodular enhancement occurred in portal venous and delayed phases.
Dr. Fu-Hua Yan, graduated from Shanghai Medical University as a postgraduate in 1996, now an associated professor, a radiologist in chief specialized in the diagnosis of CT and MRI in hepatic diseases, having 22 papers published. She is the associated editor of the books Spiral CT and Body MRI.
Edited by Ma JY
| 1. | Berloco P, Caricato M, Ripetti V, Cellamare C, Altomare V, Alloni R, Arullani A. [Multifocal inflammatory pseudotumor of the liver]. Ann Ital Chir. 1998;69:371-377. [PubMed] |
| 2. | Zamir D, Jarchowsky J, Singer C, Abumoch S, Groisman G, Ammar M, Weiner P. Inflammatory pseudotumor of the liver--a rare entity and a diagnostic challenge. Am J Gastroenterol. 1998;93:1538-1540. [PubMed] |
| 3. | Yavuz E, Büyükbabani N, Cevikbas U. Inflammatory pseudotumor of the liver. Report of two cases. Pathologica. 1998;90:463-466. [PubMed] |
| 4. | Yoon KH, Ha HK, Lee JS, Suh JH, Kim MH, Kim PN, Lee MG, Yun KJ, Choi SC, Nah YH. Inflammatory pseudotumor of the liver in patients with recurrent pyogenic cholangitis: CT-histopathologic correlation. Radiology. 1999;211:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Kelekis NL, Warshauer DM, Semelka RC, Eisenberg LB, Woosley JT. Inflammatory pseudotumor of the liver: appearance on contrast enhanced helical CT and dynamic MR images. J Magn Reson Imaging. 1995;5:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ijuin H, Ono N, Koga K, Yoshida T, Ohnishi H, Miyazaki S, Ogata H, Kohakura M, Ogawa K, Yamawaki M. Inflammatory pseudotumor of the liver--MR imaging findings. Kurume Med J. 1997;44:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Abehsera M, Vilgrain V, Belghiti J, Fléjou JF, Nahum H. Inflammatory pseudotumor of the liver: radiologic-pathologic correlation. J Comput Assist Tomogr. 1995;19:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Lim JH, Lee JH. Inflammatory pseudotumor of the liver. Ultrasound and CT features. Clin Imaging. 1995;19:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR Am J Roentgenol. 1996;167:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Krech RH, Erhardt-Domagalski M, Neumann H. [Inflammatory pseudotumor of the liver. Morphologic and cytophotometry studies and differential diagnosis]. Pathologe. 1995;16:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Zhou KR. Body MRI. 1st Ed. Shanghai: Shanghai Medical University Publisher 2000; 814. |
| 12. | Materne R, Van Beers BE, Gigot JF, Horsmans Y, Lacrosse M, Pringot J. Inflammatory pseudotumor of the liver: MRI with mangafodipir trisodium. J Comput Assist Tomogr. 1998;22:82-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |