MATERIAL AND METHODS
Cell lines and cell culture
Colon adenocarcinoma cell lines (LS174-T cells and LoVo cells, provided by the Cell Bank of Chinese Academy of Sciences) were cultured in RPMI 1640 medium at 37 °C in a humidified 5% CO2 atmosphere. Cells were seeded at a concentration of 1 × 106/well in 6-well plates and incubated at 37 °C in a humidified 5% CO2 atmosphere overnight to ensure that the cells adhere to the walls, then chemotherapeutic drug of various concentrations was added and incubated again for 24 h or 48 h.
Treatment with chemotherapeutic drugs
Different cell lines were treated with Cisplatin (CDDP), Mitomycin (MMC), 5-fluorouracil (5-FU) and epirubicin Hydrochloride (EPH) at concentrations of plasma peak concentration (PPC), 1/10 PPC, 1/5 PPC, 5 PPC and 10 PPC for 24 h and 48 h. The PPC of CDDP, MMC, 5-FU, EPH was 3 mg/L, 3 mg/L, 10 mg/L and 0.6 mg/L[5].
Apparatus and reagent
EPHCS-XL FACScan was purchased from Coulter Co. of America. Annexin-V-Fluos labeling reagent for identifying apoptosis was purchased from Boehringer Mannheim Co., and reagent for identifying Fas/FasL expression was purchased from Pharmingen Co..
Determination of apoptosis and Fas/FasL expression
Early apoptotic changes were identified using Annexin-V-Fluos, which binds to phosphati dylserine on the outer leaflet of apoptotic cell membranes. Propidium iodide was used for the discrimination of necrotic cells from the annexin V positively stained cell cluster. Cells were trypsinized, washed with PBS twice, centrifuged at 200 × g for 5 min, and resuspended in 100 mL Annexin-V-Fluos labeling solution containing Annexin-V-Fluos reagent and propidium iodide. Cells were incubated for 10-15 min and analyzed on FACScan. Percentage of apoptosis = 100% [experimental apoptosis (%)-spontaneous apoptosis in medium (%)]/[100%-spontaneous apoptosis in medium (%)][16]. For determination of Fas expression, cells were stained with anti-Fas IgG1 monoclonal antibody (mAb 1 g/mL) for 45 min at 4 °C followed by rat anti-mouse IgG1-FITC for 30 min at 4 °C. FII23 IgG3 antibody was used as isotype-matched antibody to control non-specific binding. For determination of FasL expression, cells were stained with FITC-conjugated rat anti-FasL mAb for 45 min at 4 °C followed by goat anti-mouse Igs-biotinylated. Rat IgG2a antibody was used as isotype-matched control antibody. Fas/FasL expression was assessed by FACScan. Fas expression = (%Fas + treated cells-%IgG1-FITC + treated cells)-(%Fas + control cells-%IgG1-FITC + control cells)[16]. FasL expression was calculated according to the formula as that of Fas expression. Cells were analyzed by FACScan using Cell Quest software.
DNA extration and gel electrophoresis
LS174T cells and LoVo cells of colon adenocarcinoma cell lines were added to 6-well culture plates at a concentration of 1 × 106/mL. Cell cultures without treatment, or with treatment at different PPC of above chemotherapeutic drugs were harvested from the culture plates after 24 h or 48 h, washed twice with PBS (pH7.4), and moved to the micro-centrifuging tube. Cells were pelleted at 300 ×g and the medium was removed by aspiration. Cell pellets were resuspended in 100 mL lysis buffer and incubated at 50 °C for 1 h, then mixed with 10 μL RNase and incubated at 50 °C for another 1 h. The DNA was resuspended in sample buffer and quantified by absorbance at 260 nm. Ten micrograms of DNA was applied to a 1.5% agarose gel in TBE buffer and resolved at 75 mA constant current for 2 h. The DNA was then visualized by ethidium bromide staining for 5 min and washed twice with distilled water. The result was observed under ultraviolet radiation.
Statistical analysis
Fas expression FasL expression and apoptosis percentage of each treated group were compared and checked with F test, and their relationship was analyzed.
RESULTS
The drug concentrations of CDDP, MMC, 5-Fu and EPH ranged between 1/10 PPC and 10 PPC[15], among which we set 1/5 PPC, PPC, and 5 PPC[17]. We treated LS174T cells and LoVo cells with chemotherapeutic drugs fo 24 h and 48 h respectively, and detected apoptosis and Fas/FasL expression with FACScan.
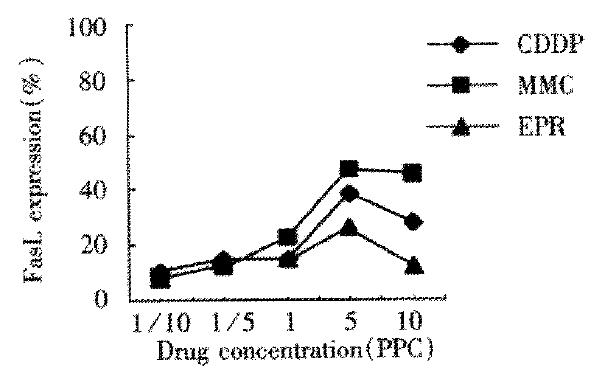
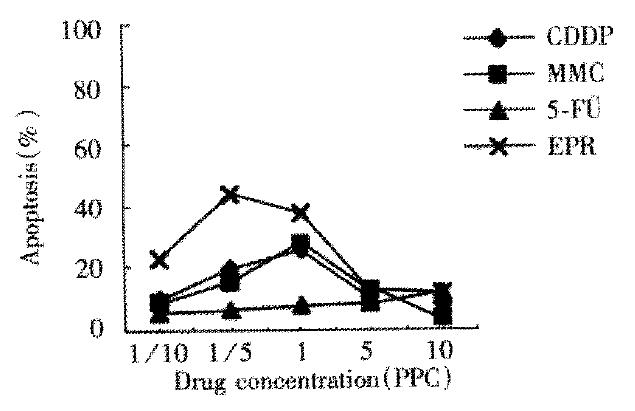
In untreated control group of LS174T cells, Fas expression, FasL expression and apoptosis percentage was 0.5%, 1.5% and 2.8% separately. The percentage in those treated with chemotherapeutic drug for 24 h was similar in that treated for 48 h, but figures were lower. LS174T cells treated with chemotherapeutic drugs for 48 h showed that Fas expression was upregulated when drug concentration was below 1/5 PPC or at PPC, and downregulated when drug concentration was above 1/5 PPC or at PPC (Figure 1). FasL expression was upregulated obviously when drug concentration was above PPC (Figure 2). When drug concentration of CDDP, MMC and EPH was below 1/5 PPC or PPC, apoptosis was upregulated, and downregulated at a concentration above 1/5 PPC or PPC. Apoptosis of 5-Fu group cells was upregulated with increase of drug concentration. Apoptosis in various drug concentrations in each group is shown in Figure 3.
Figure 1 Fas expression of LS174T cells treated with chemotherapy drugs for 48 h.
Figure 2 FasL expression of LS174T cells treated with chemotherapy drugs for 48 h.
Figure 3 Apoptosis of LS174T cells treated with chemotherapy drugs for 48 h.
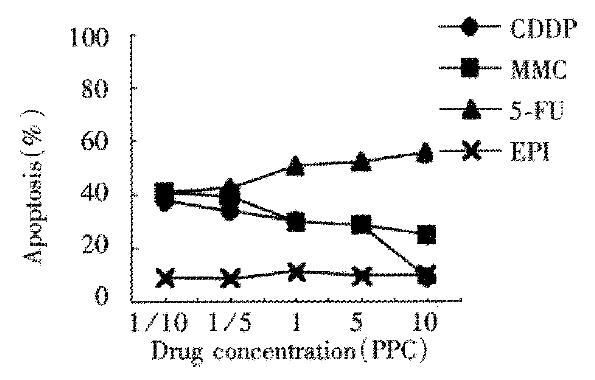
We did not detect Fas expression of LoVo cells treated with chemotherapeutic drugs for 24 h. FasL expression was lower than that of LS174T cells. Apoptosis of 5-Fu group was upregulated with ascending drug concentration which was 54.8% when drug concentration was 10PPC. The effect of apoptosis induced by 5-Fu was the best while that induced by EPH was the worst. The results were just opposite to the results in LS174T cells. Apoptosis of CDDP group and MMC group were downregulated with ascending drug concentration (Figure 4). We could not detect Fas expression or FasL expression of LoVo cells treated with chemotherapeutic drugs for 48 h. Necrosis was found mainly in LoVo cells, apoptosis of which was lower than that of the control group.
Figure 4 Apoptosis of LoVo cells treated with chemotherapy drugs for 24 h.
We abstracted DNA from the control group of cell lines and the group treated with chemotherapeutic drugs of various concentrations for 24 h and 48 h, and examined DNA ladder by agarose gel electrophoresis. The result showed that DNA ladder was remarkable in the treated group of LS174T cells when drug concentration of CDDP, MMC and EPH was 1/5 PPC or PPC, and 5-Fu concentration was 10 PPC. DNA ladder was remarkable in the treated LoVo cells when CDDP, and MMC concentration was 1/10 PPC, and 5-Fu concentration was 10PPC.There was no obvious difference among the DNA ladders at any concentration of EPH (Figure 5, Figure 6).
Figure 5 DNA ladder LS174T cells treated with chemotherapy drugs for 48 h (1.
Markers; 2. The control group; 3. 5-Fu; 4. DDP; 5. EPI; 6. MMC).
Figure 6 DNA ladder of LoVo cells treated with chemotherapy drugs for 24 h (1.
Markers; 2. The control group; 3. 5-Fu; 4. DDP; 5. EPI; 6. MMC).
DISCUSSION
Fas is a 45 kDa type I transmembrane receptor expressed by a variety of normal and neoplastic cells. It belongs to the tumor necrosis factor (TNF) receptor family. When there is no antagonistic factor in the induction of apoptosis, apoptosis of chemosensitive tumor cells is induced following cross-linking of agonistic anti-Fas antibody or FasL with functional Fas expressed by tumor cells. Chemotherapeutic drug could induce tumor cells, which did not express or weakly expressed Fas, to strongly express functional Fas. Our data by FACScan showed that the Fas expression of LS174T cells untreated with chemotherapeutic drugs was only 0.5%, but compared with the Fas expression of the control cells, the Fas expression of LS174T cells treated with CDDP, MMC, 5-Fu and EPH for 48 h was notably upregulated. Fas expression of each treated group reached its peak at 1/5 PPC or PPC, which proved that the appropriate selection of chemotherapeutic drug concentration could effectively induce functional Fas to be strongly expressed on the surface of tumor cells.
In LS174T cells, apoptosis induced by CDDP, MMC and EPH was closely related to Fas-expression. Fas expression of the four LS174T cell groups treated with chemotherapeutic drugs for 48 h and apoptosis reached their peak at 1/5PPC or PPC. Fas expression was downregulated with chemotherapeutic ascending, drug concentration, so was apoptosis. The results of DNA fragmentation studies showed that the DNA ladder was most visible when the concentration of CDDP, MMC and EPH was 1/5 PPC or PPC. Fas expression and apoptosis of CDDP, MMC and EPH groups were positively correlated by relativity analysis (P < 0.05). In LS174T cells, Fas expression induced by EPH was most notable and apoptosis of this group was the highest of all. There was statistical significance in Fas expression and apoptosis between EPH and CDDP or MMC groups (P < 0.05). The above data and analysis suggest that kinetics of cell apoptosis correspond to kinetics of Fas induction. We could find the hypersensitive drugs and their appropriate dosage according to apoptosis and functional Fas expression. The different apoptosis paths between LS174T cells and LoVo cells demonstrated the importance of individualized selection of chemotherapeutic drugs.
After treatment with 5-Fu, Fas expression of LS174T cells was remarkablely upregulated while apoptosis was the lowest of all. On the contrary, the anti-tumor effect of 5-Fu on digestive tract tumor is the best among the clinical chemotherapeutic drugs. This contradiction, we believed, may be caused by the different effects between in vitro experiment and in vivo treatment. in vitro, FasL expression of LS174T cells induced by 5-Fu was too low to effectively activate the Fas system and trigger the apoptosis cascade; in vivo, FasL expressed strongly on the surface of CTL or NK could mediate autocrine or paracrine chemosensitive cell death by crossing its cognate receptor. This may be the reason why the 5-Fu had best anti-tumor effect in digestive tract tumor. Our study also showed that apoptosis induced by 5-Fu increased with the concentration, which was different from other drugs used in this research.
In LS174T cells, the FasL expression of CDDP, MMC and EPH groups reached its peak at 5PPC, and there was no significant correlation between FasL expression and apoptosis of each group (P > 0.05). FasL was upregulated on the tumor surface upon drug incubation in chemosensitive tumor cells and tumor cells could mediate autocrine or paracrine to release soluble FasL (sFasL). Apoptosis could occur in tumor cells expressing FasL through suicide or fratricide, inducing Fas-mediated apoptosis of peripheral normal cells, CTL and NK which express Fas, to promote local tumor invasion or metastases[18]. Therefore, drug-induced expression of FasL in Fas-negative tumors is not effective and, could result in selective elimination of antitumor lymphocytes. The incorrect chemotherapy could cause drug resistance or immune privilege of tumor cells[19]. Therefore, the induction of FasL by chemotherapeutic drug has dual effects on tumor therapy.
FACScan determination showed that LoVo cells treated with chemotherapeutic drug did not express Fas, weakly expressed FasL, and there was no significant correlation between Fas expression and apoptosis in LoVo cells (P > 0.05). However, DNA ladder of LoVo cells treated with chemotherapeutic drug was similar to that of LS174T cells, suggesting that drug-induced apoptosis of LoVo cells did not depend on FasL/Fas interaction. Drug might kill tumor cells through activating other death receptors or directly acting on the downstream factor of Fas system (such as caspases family, etc.), also might trigger apoptosis in another way[20-28]. Nita et al[29] used equitoxic (IC50%) doses of 5-Fu to induce apoptosis in LoVo cells and analysed Bcl-2, Bcl-XL, Bax, Bad, Bak and p53 protein expression of LoVo cells by Western blotting, and found that Bcl-XL was expressed in all the cell lines and accompanied by increased expression of Bax and Bak. We are doing further studies to find out whether the ratio of Bcl-XL to Bax is correlated with chemosensitivity of LoVo cells treated with 5-Fu.
The determination of Fas expression, FasL expression and apoptosis of tumor cells treated with chemotherapeutic drug by FACScan indicated that the induction of Fas expression and apoptosis by major chemotherapeutic drugs was the best at the concentration of PPC or below PPC. DNA ladder also proved that the selection of appropriate dosage was of great importance in chemotherapy. We could find hypersensitive drugs and their appropriate dosage according to apoptosis determined by FACScan, partly according to Fas expression. The different apoptosis paths between LS174T cells and LoVo cells demonstrated the importance of individualized selection of chemotherapeutic drugs.