Published online Feb 15, 2001. doi: 10.3748/wjg.v7.i1.37
Revised: October 12, 2000
Accepted: October 19, 2000
Published online: February 15, 2001
AIM: To examine the expression of activin A, a member of the transforming growth factor (TGF-β) superfamily, recently has been reported to beoverexpressed in liver cirrhosis, in the course of carbon tetrachloride-induced rat hepatic fibrosis.
METHODS: Hepatic fibrosis was induced in rats by subcutaneous injections of 40% carbon tetrachloride oily solution for a period of 1 to 7 weeks. At the end of 1, 2, 3, 4, 5, 6 and 7 weeks after carbon tetrachloride injections, the rats were killed in group (6-10 rats each time) for study. The activin A messenger RNA expression and its protein localization were assessed by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry.
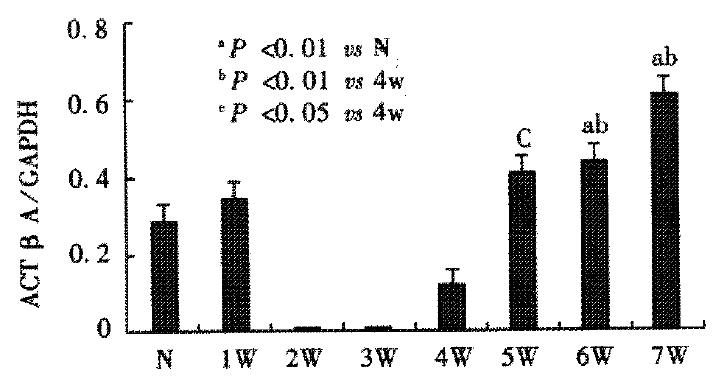
RESULTS: The normal rat liver expressed activin A mRNA and protein, and its expression was transiently decreased and became undetectable after carbon tetrachloride injections for 2 or 3 weeks and then increased gradually. After injection of carbon tetrachloride for 6 and 7 weeks, activin A mRNA and protein expressions were significantly enchanced in rat liver. Compared with that of the normal rat liver. Activin A mRNA expression levels in rats receiving carbon tetrachloride injections for 6 and 7 weeks were 1.6 and 2.2 times that of those in normal rat liver respectively (0.456 ± 0.094 vs 0.286 ± 0.0670, P < 0.01; 0.620 ± 0.134 vs 0.286 ± 0.0670, P < 0.01). Immunohistochemistry showed that activin A expressed in hepatocytes of normal liver, and its expression was decreased in rats receiving carbon tetrachloride for 2 or 3 weeks. Compared with normal liver, activin A expression distribution mode changed in fibrotic liver, being increased significantly in hepatocytes around fibrotic areas.
CONCLUSION: Activin A expression was increased in late stage of hepatic fibrosis, and this may be involved in hepatic fibrosis formation in this period.
- Citation: Huang X, Li DG, Wang ZR, Wei HS, Cheng JL, Zhan YT, Zhou X, Xu QF, Li X, Lu HM. Expression changes of activin A in the development of hepatic fibrosis. World J Gastroenterol 2001; 7(1): 37-41
- URL: https://www.wjgnet.com/1007-9327/full/v7/i1/37.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i1.37
Many researchers have tried to elucidate the pathogenesis of hepatic fibrosis[1-11]. Multiple studies have proved that cytokines play a vital role in this process, especially transforming growth factor β (TGF-β ) superfamily[12-21]. Activins are newly found multifunctional proteins that belong to the TGF-β superfamily[22]. There are three kinds of activins, A (βAβA), B(βBβB) and AB(βAβB), which are hetero/homodimers closely related β-subunits of inhibin (βA and βB)[23]. Discovered by virtue of their capacity to stimulate production of follicle-stimulated hormone (FSH) from pituitary gland, activins have widespread anatomical distribution and are implicated in the regulation of many biological processes, including proliferation and differentiation of various types of cells[24]. In liver, ACT A inhibits growth of heptocytes and induces apoptosis, but promotes mesenchymal proliferation and extracellular matrix (ECM) production, and these effects are similar to those of TGF-β[25-27]. Activins and TGF-β may implicate in development of hepatic fibrosis jointly. Sugiyama et al[27] have demonstrated that activin A expression was significantly enhanced in cirrhotic and fibrotic rat livers induced by dimethylnitrosamine (DMN) and porcine serum. However, there has been no consensus of opinion about activin expression changing mode and its cell origins in fibrotic rats liver yet, for some researchers have got different results from those of Sugiyama. By means of RT-PCR and immunohistochemstry, we have examined the activin A expression mode in carbon tetrachloride (CCl4) induced fibrotic rat liver.
Adult male Sprague Dawley rats, weighing 180 g-210 g, were obtained from Shanghai Experimental Animal Institute of the Chinese Academy of Sciences. Hepatic fibrosis rat model was created by subcutaneous injections of CCl4[28-36]. Altogether 72 rats were used. They were randomly divided into the normal control group (6 rats) and model group (64 rats). For the model group rats, CCl4 olive solution (40% CCl4) was injected subcutaneously at a dose of 0.4 mL/100 g twice a week. A double dose was used for the first injection. A same volume of saline was injected in normal control group animals. For study by lots every week (from the 1st to 7th week), 72 hours after last injection of CCl4, 6-10 model group rats were sacrificed. One portion of each liver was immediately frozen in liquid nitrogen for RNA extraction, and the remainder was fixed with 10% (vol/vol) formalin for van Gieson (VG) staining or immunostaining.
Deparaffinized 5 μm thick liver sections were washed three times with phosphate-buffered saline (PBS; pH7.4), incubated in endogenous peroxidase blocking solution (Immunostain EliVision Kit, Maxim Biotech, Inc), and then treated with 0.01 M citrate buffer pH6.0 for 10 minutes in a microwave oven at 650 W. Non-specific-antibody binding was blocked by pretreatment with PBS containing 0.5% bovine serum albumin (fraction V powder, Sigma). Sections were then rinsed in PBS and incubated overnight at 4 °C with diluted monoclonal antibody against activin βA subunit (Serotic, UK) at 1:100 in PBS followed by three washes in PBS containing 0.05% Tween-20. The steps were performed using Immunostain EliVision kit according to the manufacturer’s instructions. Sections were stained with 3,3’-diaminobenzidine tetrahydrochloride (DAB) as a chromogen. The slide was rinsed with distilled water, counterstained with hematoxylin, dehydrated, air dried, and mounted. The negative control slides were treated with nonspecific mouse IgG. The sections were examined under light microscopy.
Total RNA was extracted from liver tissues according to the guanidinium isothiocyanate single-step methods (Kit from Watson Biological Inc, Shanghai). The RNA concentration of each sample was estimated by measuring the absorbance at 260 nm.
Reverse transcription (RT) was performed using RT kit from Bioneer. The 20 μL reaction contains total RNA 2 μg, and oligo (dT)6 12.5 pmol (Sangon, Shanghai). The first-strand cDNA sample (2 μL) was added to 25 μL of a PCR reaction mixture containing 0.5 μM gene-specific primers, 2 mM MgCl2, 0.2 mM dNTP, and 1.5 units Taq polymerase, 2.5 μL 10 × buffer. Activin βA[37] sense primer: 5’-cagtcgtggacggtgcagaagt-3’; antisense primer: 5’-gcctgcggtgaggatggtctt-3’; the predicted size of the amplification products was 474 bp. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (house-keeper gene) sense primer[38]: 5’-catggtctacacgttccagt-3’; antisense primer: 5’-ggctaagcagttggtggtgc-3’; the 329 bp fragment amplification product was used as a positive control of the reverse-transcriptase receation and tissue mRNA integrity. Amplification conditions included initial denaturation for 5 minutes at 94 °C, 30 cycles of amplification for activin βA and 28 cycles for GAPDH, with denaturation at 94 °C for 45 seconds, annealing at 60 °C for 45 seconds, and extension at 72 °C for 1 minute. PCR products were analyzed by electrophoresis through agarose gel (2% wt/vol) and visualized by ethidium bromide staining and ultraviolet illumination. The length of PCR products was measured by comparison with standard molecular-weight marker. The signal intensity of each band was normalized by comparing it with the intensity of GAPDH.
Results were expressed as mean ± SE. To compare the mean values, ANOVA analysis was applied. Differences were considered significant if P < 0.05.
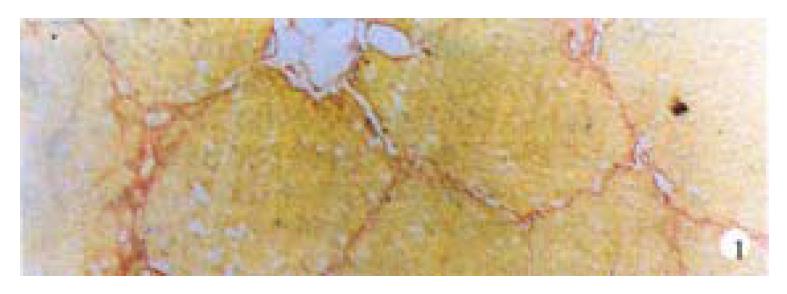
Hepatic fibrosis gradually developed with increasing CCl4 injections. After 6-7 weeks, VG staining showed that liver from most of model group animals were forming fake liver lobuli with reticulin fibers spreading radially between the portal tracts and central veins (Figure 1).
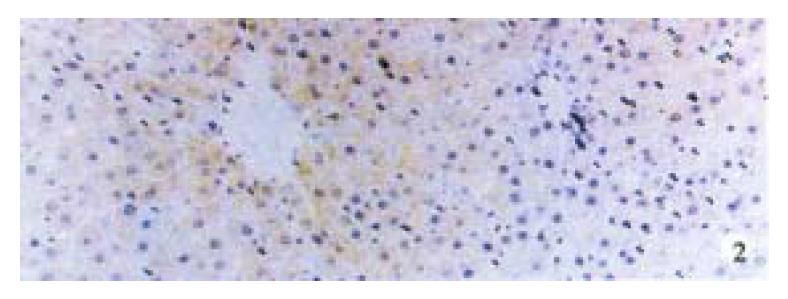
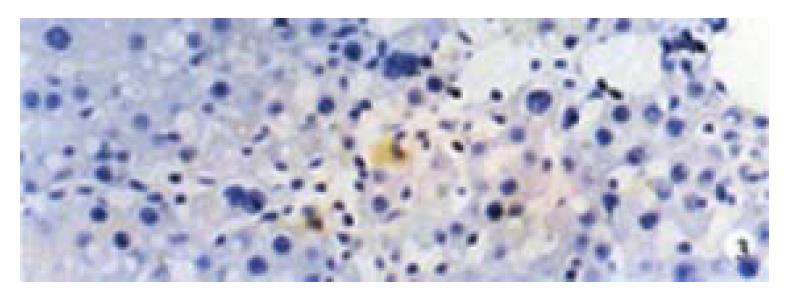
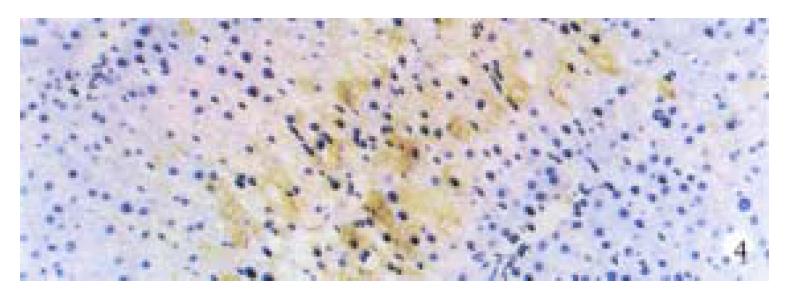
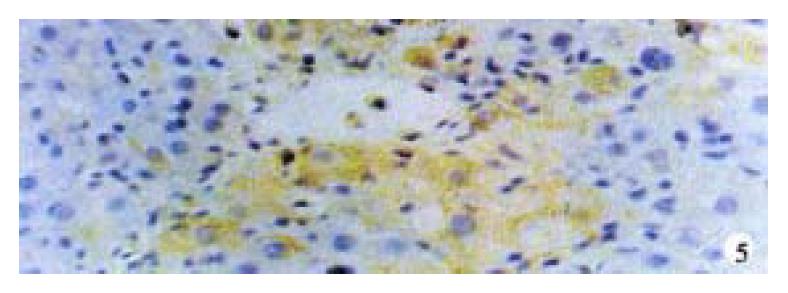
Activin βA subunit was detected in normal liver. Figure 2 shows that activin βA immunoreactivity was distributed within the lobuli and localized in parenchymal cells. More staining was found in hepatocytes around central veins. No staining was found in hepatocytes around portal tracts. After CCl4 in jection for 2-3 weeks, activin βA immunostaining was decreased but still detectable, and its distribution was changed, localizing mainly in hepatocytes around fibrotic areas (Figure 3). After CCl4 injection for 6-7 weeks, activin βA immunostaining was enhanced. Many hepatocytes around fibrotic areas (around portal tracts, fibrotic septa and central veins areas) were stained positive (Figure 4, Figure 5).
Activin βA mRNA subunit was detected in normal rat liver, but it became undetectable after CCl4 was injected for 2-3 weeks. Four weeks after CCl4 injections, activin βA mRNA levels increased gradually, and were significantly enhanced after 6 or 7 weeks when compared with normal rat liver (P < 0.01) (Figure 6, Figure 7). Activin βA mRNA levels in rats treated with CCl4 for 6 and 7 weeks were 1.6 and 2.1 times of those in normal rat liver respectively.
Liver cirrhosis is a final stage of various chronic liver diseases and is characterized by a marked deposition of extracellular matrix, combined with impared hepatic regeneration. Several cytokines are involved in the pathogenesis of this disease[39]. In particular, TGF-β1 is overexpressed in nonparenchymal cells of fibrotic liver of human or animals, and plays a pivotal role in the development of cirrhosis by stimulating fibroblast proliferation and ECM secretion[40-44]. Recent studies showed that activin may also be involved in the development of cirrhosis.
Activin A has mutiple effects including proliferation and differentiation of various types of cells[24]. In terms of its effects on hepatocytes, activin A inhibits DNA synthesis and induces apoptosis of hepatocytes[25,26,45]. In fibrotic or cirrhotic rat liver induced by CCl4, De Bleser found that activin A disappeared in parenchymal cells, and it was expressed when the liver was normal. The normal hepatic stellate cells showed no activin A expression, but its expression was significantly enhanced when hepatic fibrosis had formed[17,46]. Subsequently, Sugiyama[27] found activin A expression was greatly increased in fibrotic rat liver induced by DMN and porcine serum, but it was in hepatocytes but not hepatic stellate cell that activin A expression was significantly increased. Obviously, results from De Bleser and Sugiyama were not completely consistent with each other. Our research showed that activin A expression was increased in CCl4-induced fibrotic rat liver, being localized in hepatocytes. These results were consistent with those of Sugiyama.
We had used the same hepatic fibrosis model as De Bleser’s, but the results were different. This may be due to a difference in the period of model-making. We found that normal liver expressed activin A, but after injection of CCl4 for 2-3 weeks, activin A mRNA became undetectable, although its protein could still be found. Four weeks later, activin A mRNA increased gradually. But it was not significantly increased until CCl4 was injected for 6 or 7 weeks when hepatic fibrosis had formed completely. This means that most of livers have formed pseudo lobuli. Sugiyama also found that in the early period of hepatic fibrosis induced by DMN, the expression of activin βA mRNA was discreased transiently, and then increased gradually with lapse of time. However, the expression was enhanced continuously in porcine serum-induced cirrhosis. This difference may be due to serious hepatocytic damage caused by CCl4, which is also found in the early stage of the development of DMN induced liver cirrhosis but not in porcine serum induced cirrhosis[47-50]. As activin A acts as an autocrine growth inhibitor of hepatocytes, this transient decrease in expression may promote hepatocytic regeneration. De Bleser reported that activin βA expression disappeared in parenchymal cells of CCl4 induced cirrhosis, but we think that it might be a transient disappearance, for liver cirrhosis was not formed completely. In addition, De Bleser found that activin A expressed in activated hepatic stellate cells (HSC) of fibrotic liver, but according to our result, activin A immunostaining was mainly distributed in hepatocytes around fibrotic areas (Figure 4, Figure 5), a result also consistent with Sugiyama’s reports. So it is worthy of further study to confirm whether HSC expresses activin A or not.
The expression of activin A protein was not complete in conformity with its mRNA. Its mRNA became undetectable after CCl4 injections for 2 to 3 weeks, but activin A protein could be detected by immunohistochemistry. Sugiyama described similar phenomenon in DMN-induced cirrhosis. This may be because of the fact that the mRNA level was too low to be detected by our method. Of course, other reasons may exist and worth further studying.
In summary, our study shows that in CCl4-induced rat cirrhosis, the expression of activin A is decreased transiently at first, and then increased gradually. Activin A may be involved in the late stage of the development of liver cirrhosis. Distribution of activin A also changes with the development of fibrosis, that is from hepatocytes around central veins to hepatocytes around fibrotic areas including central vein, connective septa and portal tracts.
Edited by Ma JY
| 1. | Yan JC, Liu JH, Ma Y. Vascular obstruction in pathogenesis of hepatitis B fibrosis. Xin Xiaohuabingxue Zazhi. 1997;5:647-649. |
| 2. | Jia JB, Han DW, Xu RL, Gao F, Zhao LF, Zhao YC, Yan JP, Ma XH. Effect of endotoxin on fibronectin synthesis of rat primary cultured hepatocytes. World J Gastroenterol. 1998;4:329-331. [PubMed] |
| 3. | Liu CH, Hu YY, Wang XL, Liu P, Xu LM. Effects of salvianolic acid-A on NIH/3T3 fibroblast proliferation, collagen synthesis and gene expression. World J Gastroenterol. 2000;6:361-364. [PubMed] |
| 4. | Chang XM, Xue H, Duan ZB, Feng XL, Jia AL. Diagnostic value of simutaneous determination of serum protocollagen III and hyalu-ronic acid in patients with chronic liver diseases. Xin Xiaohuabingxue Zazhi. 1994;2:223-224. |
| 5. | Liu SR, Gu HD, Li DG, Lu HM. A comparative study of fat storing cells and hepatocytes in collagen synthesis and collagen gene expression. Xin Xiaohuabingxue Zazhi. 1997;5:761-762. |
| 6. | Pan XF, Zhang CF, Qiu WW, Lu GQ, Zhou MW, Chang J, Ni Y, Li SQ, Han TQ. Alterations of serum laminin and hyaluronic acid in patients with chronic liver diseases. Xin Xiaohuabingxue Zazhi. 1996;4:373-374. |
| 7. | Gao CF, Kong XT, Fan LY. Isolation, culture and identification of lipocytes and Kupffer cells in rats. Xin Xiaohuabingxue Zazhi. 1995;3:8-10. |
| 8. | Yang SM, Fang DC, Feng HF, Chen GM, Gao LQ. Diagnostic value of serum prolidase, procollagen type III and hyaluronic acid levels for liver fibrosis. Xin Xiaohuabingxue Zazhi. 1995;3:27-28. |
| 9. | Gu SW, Luo KX, Zhang L, Wu AH, He HT, Weng JY. Relationship between ductule proliferation and liver fibrosis of chronic liver disease. Shijie Huaren Xiaohua Zazhi. 1999;7:845-847. |
| 10. | Yan JC, Chen WB, Liu JH, Ma Y, Xu CJ. Immunohistochemical studies on intrahepatic vascular proliferation in hepatitis B. Huaren Xiaohua Zazhi. 1998;6:780-782. |
| 11. | Yan JC, Ma Y, Chen WB, Shun XH. Dynamic observation on liver fibrosis and cirrhosis of hepatitis B. Huaren Xiaohua Zazhi. 1998;6:699-702. |
| 12. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [PubMed] |
| 13. | Kogure K, Zhang YQ, Shibata H, Kojima I. Immediate onset of DNA synthesis in remnant rat liver after 90% hepatectomy by an administration of follistatin. J Hepatol. 1998;29:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Li XQ, Zeng MX, Ling QH. Effects of interferon-B on DNA synthesis and collagen production of cultured rat hepatocytes. Huaren Xiaohua Zazhi. 1998;6:488-490. |
| 15. | Huang X, Li DG, Wang ZR, Wei HS, Cheng JL, Zhan YT, Xu QF, Lu HM. Follistatin on hepatic fibrotic rat liver PCNA, BrDU and activin A expression. Shijie Huaren Xiaohua Zazhi. 2000;8:993-997. |
| 16. | Wang X, Chen YX, Xu CF, Zhao GN, Huang YX, Wang QL. Relationship between tumor necrosis factor-alphaand liver fibrosis. World J Gastroenterol. 1998;4:18. [PubMed] |
| 17. | De Bleser PJ, Niki T, Xu G, Rogiers V, Geerts A. Localization and cellular sources of activins in normal and fibrotic rat liver. Hepatology. 1997;26:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Zhang YQ, Mashima H, Kanzaki M, Shibata H, Kojima I. Assessment of the role of activin A and transforming growth factor beta in the regulation of AML12 cell growth. Hepatology. 1997;25:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci USA. 1999;96:2345-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Liu F, Liu JX, Cao ZC, Li BS, Zhao CY, Kong L, Zhen Z. Relation-ship between TGF-β 1, serum indexes of liver fibrosis and hepatic tissue pathology in patients with chronic liver diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:519-521. |
| 21. | Kogure K, Zhang YQ, Maeshima A, Suzuki K, Kuwano H, Kojima I. The role of activin and transforming growth factor-beta in the regulation of organ mass in the rat liver. Hepatology. 2000;31:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Eto Y, Tsuji T, Takezawa M, Takano S, Yokogawa Y, Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987;142:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 282] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Hübner G, Alzheimer C, Werner S. Activin: a novel player in tissue repair processes. Histol Histopathol. 1999;14:295-304. [PubMed] |
| 24. | Woodruff TK. Regulation of cellular and system function by activin. Biochem Pharmacol. 1998;55:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Hully JR, Chang L, Schwall RH, Widmer HR, Terrell TG, Gillett NA. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology. 1994;20:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Zhang YQ, Kanzaki M, Mashima H, Mine T, Kojima I. Norepinephrine reverses the effects of activin A on DNA synthesis and apoptosis in cultured rat hepatocytes. Hepatology. 1996;23:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Sugiyama M, Ichida T, Sato T, Ishikawa T, Matsuda Y, Asakura H. Expression of activin A is increased in cirrhotic and fibrotic rat livers. Gastroenterology. 1998;114:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Sun ZQ, Wang YJ, Quan QZ, Han GY, Jin XH. Change of serum phosphonate esterase in hepatic fibrosis in rats. Xin Xiaohuabingxue Zazhi. 1994;2:206-207. |
| 29. | Han GY, Jin XH, Wu CQ, Wang HF, Liu WJ. Alteration of serum phosphonate esterase in liver fibrosis in rats. Xin Xiaohuabingxue Zazhi. 1996;4:369-370. |
| 30. | Li BS, Wang J, Zhen YJ, Liu JX, Wei MX, Sun SQ, Wang SQ. Experimental study on serum fibrosis markers and liver tissue pathology and hepatic fibrosis in immuno-damaged rats. Shijie Huaren Xiaohua Zazhi. 1999;7:1031-1034. |
| 31. | Wang Y, Gao Y, Huang YQ, Yu JL, Fang SG. Gelatinase A proen-zyme expression in the process of experimental liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:165-167. |
| 32. | Yan JC, Ma Y, Chen WB, Xu CJ. Dynamic observation on vascu-lar diseases of liver tissues of rats induced by CCla-4. Shijie Huaren Xiaohua Zazhi. 2000;8:42-45. |
| 33. | Sun DL, Sun SQ, Li TZ, Lu XL. Serologic study on extracellular matrix metabolism in patients with viral liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 1999;7:55-56. |
| 34. | Hu YY, Liu C, Liu P, Gu HT, Ji G, Wang XL. Anti-fibrosis and anti-peroxidation of lipid effects of Fuzhenghuayu decoction on rat liver induced by CCla-4. Xin Xiaohuabingxue Zazhi. 1997;5:485-486. |
| 35. | Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206-209. [PubMed] |
| 36. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] |
| 37. | Woodruff TK, Meunier H, Jones PB, Hsueh AJ, Mayo KE. Rat inhibin: molecular cloning of alpha- and beta-subunit complementary deoxyribonucleic acids and expression in the ovary. Mol Endocrinol. 1987;1:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Sonoyama K, Rutatip S, Kasai T. Gene expression of activin, activin receptors, and follistatin in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G89-G97. [PubMed] |
| 39. | Kovalszky I, Nagy P, Szende B, Lapis K, Szalay F, Jeney A, Schaff Z. Experimental and human liver fibrogenesis. Scand J Gastroenterol Suppl. 1998;228:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Date M, Matsuzaki K, Matsushita M, Tahashi Y, Sakitani K, Inoue K. Differential regulation of activin A for hepatocyte growth and fibronectin synthesis in rat liver injury. J Hepatol. 2000;32:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Yasuda H, Mine T, Shibata H, Eto Y, Hasegawa Y, Takeuchi T, Asano S, Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993;92:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Ohga E, Matsuse T, Teramoto S, Katayama H, Nagase T, Fukuchi Y, Ouchi Y. Effects of activin A on proliferation and differentiation of human lung fibroblasts. Biochem Biophys Res Commun. 1996;228:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Zhang YQ, Kanzaki M, Shibata H, Kojima I. Regulation of the expression of follistatin in rat hepatocytes. Biochim Biophys Acta. 1997;1354:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Qiu DK, Li H, Zeng MD, Li JQ. Effect of cordyceps polysaccharidesliposome on the expression of interstitial mRNA in rats with hepatic fibrosis. Xin Xiaohuabingxue Zazhi. 1997;5:417-418. |
| 47. | Sun ZQ, Wang YJ, Quan QZ, Liu XF, Pan X, Jiang XL. Prevention and treatment action of tetrandrine on experimental liver fibrosis in rats. Xin Xiaohuabingxue Zazhi. 1994;2:19-20. |
| 48. | Wang YJ, Sun ZQ, Quan QZ, Zhang ZJ, Liu XF, Jiang XL, Pan X. Study on type I collagen and fat-storing cells in fibrotic rat liver treated by tetrandrine. Xin Xiaohuabingxue Zazhi. 1994;2:78-79. |
| 49. | Gong AY, Ma XH, Xu RL, Yin L, Chen XM, Zhao YC, Han DW. Changes of laminin in the liver of rats with experimental cirrhosis. Xin Xiaohuabingxue Zazhi. 1997;5:3-5. |
| 50. | Li BS, Wang J, Zhen YJ, Wang XG, Sun YH, Wang SQ, Wu ZQ. Blocking effect of Chinese herbs Yiganxian and PHGF on immuno-damaged hepatic fibrosis in rats. Huaren Xiaohua Zazhi. 1998;6:786-788. |