INTRODUCTION
Development of drug-resistance to chemotherapy and subsequent metastasis of tumor are primarily responsible for treatment failure and the death from cancer. There have been many previous studies on the relationship between expression of multidrug resistance (MDR) phenotype P-glycoprotein (P-gp) and the malignant properties of tumors, but the results are often conflicting[1-8]. The difference in tumor types or MDR phenotype induced by specific agents might account for this discrepancy. Taxotere (TXT), a member of the family of taxanes, has antitumor activity through its effect of promoting the polymerization of tubulin[9,10]. Since microtubules are involved in many respects of cell functions, such as cell movement, intracellular protein translocation, an altered invasive ability has been confirmed in in vitro invasion assay[11-14] and in vivo metastasis assay[15] in tumor cells treated with taxane. Taxane has also been found to down-or up- regulate the expressions of metastasis-associated proteins or genes, such as metallo-poteinase[13,16-18], urokinase-type plasminogen activator[18], and E-cadherin[19]. Our previous work demonstrated that the intrinsic and acquired resistance to TXT in pancreatic adenocarcinoma (PAC) cell line SUIT-2 was mediated mainly by P-gp[20]. This study demonstrated that the invasive ability of TXT-resistant cells S2/TXT was not significantly greater than that of SUIT-2. However, S2/TXT cells have increased resistance to the anti-invasion effects of TXT as compared with their parental cells, SUIT-2.
MATERIALS AND METHODS
Reagents
Taxotere, obtained from Rhone-Poulenc Rorer Pharmaceuticals Inc., was stored as 10 mM stock solution in absolute ethanol at -20 °C. This solution was further diluted in the medium and used in the cell culture immediately before each experiment. MTT (3-[4,5dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) was dissolved in phosphate buffered saline (PBS) at a concentration of 5 g/L and filtered. The MTT solution was stored in the dark at 4 °C before use.
Cell cultures
Human pancreatic cancer cell line SUIT-2 was established by Iwamura et al[21]. This cell line was derived from a metastatic liver tumor of human moderately differentiated pancreatic carcinoma. Its sublines including S2-007, S2-013, S2-020 and S2-028 were cloned by soft agar culture and showed different metastatic potential[22]. Cells were cultured in plastic flasks with McCoy’s modified medium supplemented with 10% fetal bovine serum (FBS) (Sigma) and 1.25% penicillin-streptomycin solution (Sigma) (designated as “culture medium” below) and maintained at 37 °C in humidified atmosphere containing 5% CO2.
Development of TXT resistant SUIT-2 cell line (S2/TXT)
A SUIT-2 TXT resistance derivative was developed by growing the parental cell line SUIT-2 in increasing concentration of TXT. Initially, the cells were grown as monolayers in culture medium at 37 °C in 5% CO2 humidity atmosphere and exposed to 0.1 nM TXT with addition of fresh culture medium and drugs every 3 d. After 2 weeks, the cells were exposed sequentially to stepwise increasing concentration of the drug until a TXT concentration of 2 nM was achieved. These cells were maintained in a drug-free culture medium for at least 3 weeks before used in experiments.
MTT colorimetric assay
The MTT colorimetric assay was performed as described by Page et al[23]. Briefly, SUIT-2 and S2/TXT cells were grown within 96 well microtitre plates (Costar) at 2 × 104 cells/100 µL of culture medium per well and acclimated for 6 hours. Various concentrations of drugs (100 µL) diluted in culture medium were added. Five duplicate wells were used for each determination. The plates were incubated at 37 °C in 5% CO2 for 72 hours when the control cells reached 90% confluence, and 30 µL of MTT solution was then added to each well and the plates were incubated at 37 °C for another 4 h. The medium and MTT solutions were then aspirated and 150 µL of dimethyl sulfoxide (DMSO) (Sigma) was added. The plates were agitated on the shaker for 15 minutes and read on Bio-Tek Microplate reader EL 800 (Bio-Tek Instruments, Inc.) with DeltaSoft 3 software. Fraction of cell proliferation was defined as the ratio of optical density volume to that of controls. The IC50 was defined as the concentration of the drugs required to reduce the optical density by 50% in treated cells to that of the controls.
Reverse transcription-polymerase chain reaction (RT-PCR)
The isolation of total RNA was based on the method of Chomczynski and Sacchi[24]. After the SUIT-2 and S2/TXT cell lines grew to 90% confluence, the total RNAs were extracted from the cell lines using Trireagent (Biotechnique, Molecular Research Center, Inc.). The total RNA was also isolated from the SUIT-2 cells incubated with 0.4 nM of TXT for 24 h. The messenger RNA was quantitated by measuring its absorbance at 260 nm. Equal amounts of RNA were reversed transcribed using SuperScriptTM One-stepTM RT-PCR System (Life Technologies). The 25 µL PCR mixed in each tube containing 0.5 µL RT/Tag Mix, 3 µL of 5 mM MgSO4, 5 µL diethy pyrocarbonate (DEPC, Sigma) treated distilled water, 3 µL mixed primer pairs, 12.5 µL 2X reaction mix and 1 µg template RNA in DEPC water. After an initial denaturation in a programmable thermocycler at 94 °C for 2 minutes, PCR was carried out for 30 cycles with the thermal profile: denaturing at 94 °C for 30 seconds, annealing at 55 °C for 30 seconds and extension at 72 °C for 1 minute with an extra 10 minutes extension for the last cycle. After completion of the amplification cycles, 5 µL of each PCR product was electrophoresed at 60 V for 1.5 h on a 1.2% agarose gel (GIBCOBRL) in Trizma base and glacial acetic acid EDTA buffer, together with a 100-bp DNA (GIBCOBRL). The specific primers for mdr1 used in this study were sense 5’-CCCATCATTGCAATAGCAGG-3’, antisense: 5’-GTTCAAACTTCTGCTCCTGA-3’. The metastasis of the carcinoma involved many kinds of genes. The primers used to detect the metastasis-related genes in this study were MMP-2: sense 5’-GAGCTGAAGGACACACTAAAGAAGA-3’; antisense 5’-TTGCCATCCTTCTCAAAGTTGTAGG-3’, MMP-9: Sense 5’-CACTGTCCACCCCTCAGAGC-3’; antisense 5’-GCCACTTGTCGGCGATAAGG-3’, Intigrin α5: sense 5’-CATTTCCAAGTCTGGGCCAA-3’; antisense 5’-TGGAGGCTTGAGCTGAGCTT-3’, intigrin β1: sense 5’-TGTTCAGTGCAGAGCCTTCA-3’; antisense 5’-CCTCATACTTCGGATTGACC-3’ and E-Cadherin: sense 5’-GTGACTGATGCTGATGCCCCCAATACC-3’; antisense 5’-GACGCAGAATCAGAATAAGAAAAGCAAG-3’. β-actin was used as controls. Its sense primer was: 5’-TGACGGGGTCACCCACACTGTGCCCATCTA-3’; antisense primer was: 5’-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3’.
Flow cytometry for Rhodamine-123 (Rho-123) accumulation assay
SUIT-2 and its subline S2/TXT cells were harvested in logarithmic growth phase with 0.25% trypsin and resuspended in phenol red-free DMEM medium at 1 × 106 cells/mL. For Rho-123 accumulation assay, aliquots of 1 mL cell suspension were preincubated with or without 5 µM Verapamil for 45 minutes at 37 °C. Rho-123, 200 µg/L dissolved in DMEM, was added and incubated for 40 minutes at 37 °C in the dark. After incubation, cells were washed twice with and resuspended in ice-cold Rho-123 free DMEM with 5 µM Ver. The accumulation of Rho-123 in cells was analyzed with flow cytometry. Ten thousand cells per sample were analyzed. The fluorescence was measured on a logarithmic scale of 4 decades of log. These cell lines, which had not been exposed to Rho-123, were used to determine the background of autofluorescence under this condition.
Fibroblast conditioned medium (FCM)
The FCM was obtained by incubating NIH 3T3 cells (ATCC) in a serum free medium. Briefly, after NIH 3T3 cells grew to 70%-80% confluence in 10% FBS McCoy’s medium, the medium was changed to DMEM containing ascorbic acid (50 mg/L), the cells were then incubated at 37 °C for 24 h. The medium was collected after spinning down the cells and stored at -80 °C[25].
Cell invasion assay
Matrigel invasion ability of cells was assayed using a Transwell cell culture chamber[25] with an 8 µm pore size polyvinylpyrrolidone-free polycarbonate filter (Costar, Cambridge, MA). At first, the filter was coated with 200 µL Matrigel (Invitrogen, 0.25 µg/µL) and allowed to air-dry overnight. The Matrigel was reconstituted the following day with 200 µL DMEM at room temperature for 30 minutes. SUIT-2 and S2/TXT cells were harvested by trypsinization and resuspended in the culture medium at concentration of 2 × 105 cells/mL. Single cell suspension (400 µL) were placed in the upper chamber of the Transwell in the presence or absence of 0.4 nM of taxotere. The lower chamber contained 1 mL conditioned medium. After the cells were incubated at 37 °C for 24 h, the cells on the upper surface of the filter were removed by wiping with a cotton swab. The filter was fixed with 3% glutaraldehyde, stained with Hematoxylin. Cells which had invaded to the lower surface of the filter in five microscopic fields of 150 × magnification, were counted in each filter. Triplicate samples were conducted. The data were expressed as the average cell number of 15 fields.
Statistics
The significance of different invasion ability between SUIT-2 and S2/TXT, before and after treatment with TXT, was analyzed with Student’s t test. Values were expressed as mean ± SD.
RESULTS
The sensitivity of SUIT-2 and S2/TXT cells to TXT
The acquired TXT resistant cell line S2/TXT was established from SUIT-2 by culturing with stepwise increasing concentrations of TXT as described in the Materials and Methods. Its IC50 (8.1 nM) was 9.5 folds that of its parental cell line SUIT-2 (IC50: 0.85 nM). No change of sensitivity to TXT was found in this cell line during 4 months of study. The doubling time and morphology were similar to that of its parental cell line SUIT-2.
Expressions of mdr1 and other metastasis related genes
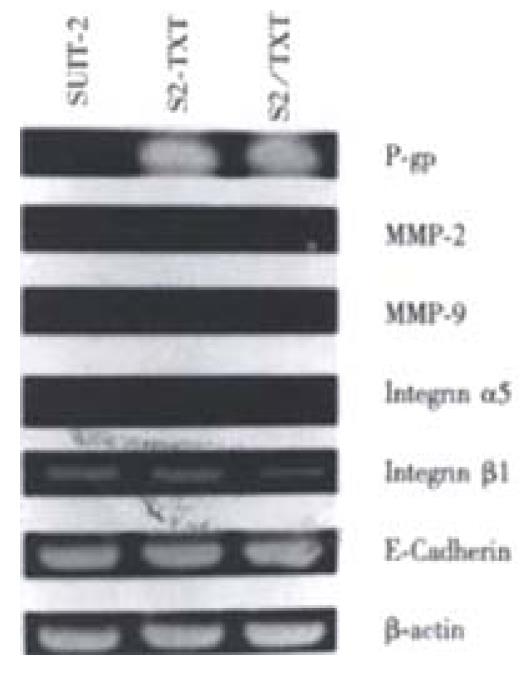
The expressions of the major drug transporter pump gene mdr1 were studied by RT-PCR. There were strong expressions of mdr1 in TXT-resistant cell line S2/TXT and SUIT-2 cells treated with 0.4 nM TXT for 24 h, and no expressions in the parental cell line SUIT-2, which were sensitive to TXT. Expression of metastasis-related genes in S2/TXT, including MMP-2, MMP-9, Integrin α5, Integrin β and E-Cadherin, was different from the parental cell line SUIT-2. Incubated with 0.4 nM of TXT for 24 h, TXT did not up- or down- regulate these metastasis-related gene expressions (Figure 1).
Figure 1 Reverse transcription-polymerase chain reaction (RT-PCR) analysis of drug resistance gene messenger RNA mdr1 in SUIT-2 and S2/TXT cell lines.
RT-PCR was performed with 30 cycles of PCR amplification. The β-actin gene RT-PCR products confirms that intact mRNA is equally present in each of the cell lines. This figure shows that there were strong expressions of mdr1 in TXT-resistant cell line S2/TXT and SUIT-2 cells treated with 0.4 nM TXT for 24 h, no expressions in the parental cell line SUIT-2. Metastasis-related gene expressions had no difference in these cells.
Rho-123 accumulation assay
Transporter activity of P-gp in S2/TXT cells was assayed by accumulation and efflux of Rho-123 tested with flow cytometry. The accumulation of Rho-123 in SUIT-2 cells is much higher than that of S2/TXT cells. The addition of 5 µM Ver significantly elevated the intracellular Rho-123 level in the TXT-resistant SUIT-2/TXT cells but not in the TXT-sensitive SUIT-2 cells.
Invasion assay
The effects of TXT on the in vitro invasion ability of SUIT-2 and S2/TXT were examined using an invasion assay system with reconstituted Matrigel membrane. The invasion ability of S2/TXT, which expressed P-gp, was not significantly different from that of SUIT-2 (83 ± 28 vs 71 ± 22 cells per field, P > 0.05). Treatment of the cells with 0.4 nM of TXT for 24 h significantly inhibited the cell invasion of SUIT-2 through the Matrigel basement membrane (71 ± 22 vs 17 ± 5 cells per field, P < 0.01). However, the TXT, in the concentration tested, had no effect on invasion of drug resistant cell line S2/TXT (83 ± 28 vs 68 ± 24 cells per field before and after TXT treatment, P > 0.05).
DISCUSSION
Pancreatic adenocarcinoma (PAC) currently remains one of the leading causes of cancer death throughout the world. Most patients are surgically unresectable at the time of diagnosis. For those who were resected, the risk of recurrence was extremely high[26]. Consequently, chemotherapy is an important approach for most patients with PAC. Taxane as a promising antitumor agent has been widely used in treatment of cancers. Unfortunately, the initial response to this agent may be hampered by the development of multidrug-resistant cells[27] and possible enhancement of malignant potential[28]. Taxane resistance of tumor cells may involve many mechanisms, but were most often related to the expression of P-gp[20]. In this study, examination of the TXT effect on the in vitro proliferation capacity of SUIT-2 and S2/TXT cells revealed a higher sensitivity of SUIT-2 when compared to the S2/TXT variant. TXT challenge of the initially drug sensitive parental SUIT-2 cell lines resulted in the development of multidrug resistance together with simultaneous expression of P-gp. Active drug transporter pump P-gp in these TXTª²resistant cells was confirmed by Rhodamine accumulation assay. However, in a study by Dumontet et al[29], only 44% of resistant clones were found to express the mdr1 gene, and studies with labeled paclitaxel (Taxol, PTX) did not show altered accumulation in mdr1 negative clones. Other studies on the mechanisms of Taxane-resistance included the composition and the mutations in β-tubulin isotypes[29,30]. The different expression of P-gp or tubulin mutation might be related to the means by which the resistant cells were selected[31]. Multi-step selected cells often present a high level of Taxane-resistance mediated by P-gp, while single-step selection yields a low level Taxane-resistance cells with tubulin mutations[31]. Since severe tubulin mutations are very likely to affect cell survival, these cells will generally be lost during the selection.
As shown in this study, TXT had marked inhibitory effects on tumor cell invasion. These effects were also found in other tumor cells treated with taxol[11-13,32]. The basic effects of Taxane on tumor cell promoted the polymerization of tubulin and stabilizing microtubule assembly, thereby blocking cell replication in the late G2 mitotic phase of the cell cycle[10,33,34]. Since microtubules are also important components of cell motility and intracellular transport[19,35,36], it is possible that TXT inhibits the invasive and migratory ability through interference with the function of the fundamental part of the cytoskeleton, such as inducing rearrangements or changes of the microtubules, which might interfere with their functional ability to mediate cell movement and protease vesicle transports and secretions of the gelatinase[13]. Direct observation by microinterferometry demonstrated that taxol can suppress the mean area of protrusion and retraction of cells and reduced the spread of cell translocation[35]. The prevention of microtubule depolyme rization by taxol can freeze the cell in a spread conformation, thereby blocking motility[37]. In some cell types, microtubules are known to serve as tracks to transport vesicles and organelles[38]. Mark et al reported that relatively low levels of taxol can inhibit secretion of the Mr 72000 and Mr 92000 type IV collagenases plus an Mr 57000 glatinase by blocking the cytoplasmic processing and packaging of the protease and completely inhibit cell attachment to matrigel, type IV collagen and plastic substrates in vitro. This has been shown to contribute to the reduced in vitro invasive ability and the establishment, growth and long-term survival of prostate tumor cells in SCID mice[13]. TXT did not, however, increase Integrin-mediated cell adhesion and cell spreading, which are attributable to microtubule depolymerization induced by microtubule disrupting agents[39].
Like other neoplasms, this pancreatic carcinoma cell line SUIT-2 is heterogeneous and consists of multiple subpopulations of cells with different invasive and metastatic properties[22]. These cells may be heterogeneous with regard to their sensitivity to chemotherapeutic agent and may contain different expressions of drug resistant phenotypes. Our previous studies have shown that the most TXT-resistant cell line in SUIT-2 and its subline (S2-007, S2-013, S2-020, S2-028) was S2-020 with strong expression of P-gp[21]. Although an in vitro study showed that S2-020 was also the most invasive toward Matrigel among these cell lines[22], there was no direct evidence that the expression of P-gp in this cell lines is responsible for its high invasive potential. On the contrary, it has been demonstrated that type I and IV collagenolytic activities are related to the malignancy of these cell lines[22,40].
The existence of different subpopulations in tumor increases the chance that cells with a high probability of survival will be selected when environmental conditions change. When SUIT-2 cell lines were treated with TXT, the highly invasive S2-020 with positive P-gp expression would be selected. However, the morphology of S2/TXT is totally different from that of S2-020 and in vitro invasive ability and morphology are similar to that of SUIT-2. Therefore, the expression of P-gp in S2/TXT was not due to the selection effect of TXT but to creation of TXT-resistant clone caused by potential of genetic mutation of TXT. In addition to the development of MDR, exposure tumor cells to some chemotherapeutic agents can cause activation or inactivation of genes with altered behavioral phenotype[41]. There was, however, no evidence that this occurred in this specific cell line treated with TXT, as shown by analysis of a number of metastasis-related genes. Thus, TXT appears capable of inducing the expression of P-gp without changing the intrinsic malignant characteristics of SUIT-2 cells.
In contrast to the study by Belotti et al[11], which show the PTX inhibits the motility of parental and PTX resistant cells equally, TXT did not inhibit the invasiveness of the TXT-resistant cell SUIT-2. This difference might be related to the mechanisms of taxane-resistance. Although the effect of PTX on cell motility and invasiveness is independent of its effect on cell proliferation[11], these effects might still be based on the intracellular concentration of the drug. Studies have shown that the tumor cells can present TXT-resistance with normal intracellular concentration of TXT. This kind of resistance usually is mediated by changes of beta tubulin isotypes[31]. In such cases, the normal intracellular drug concentration in the TXT-resistance cell can be reached as in the TXT sensitive cells. In the S2/TXT cell line, which expresses active drug transporter pump P-gp, the intracellular concentration of TXT might not be high enough to act on microtubule and to inhibit cell invasion. It indicates that the resistance to the cytotoxic activity of TXT also confers resistance to the anti-invasion of the drug in the cell line SUIT-2. Therefore, the collateral anti-tumor effects of chemotherapeutic agents, as proposed by other studies[13], will disappear in this TXT-resistant cell line when treated with taxotere.
The relationship between P-gp expression and tumor malignancy is controversial. Although some studies have found that the expression of P-gp is casually related to a less aggressive pheno-type[4,42,43], in numerous cases, metastases exhibit a multidrug resistant pattern. Clinical and in vitro studies have also provided correlative results concerning the changes of metastatic potential following acquisition of the MDR phenotype[2,3,8,44]. To date, there has been no direct evidence showing that P-gp is involved intumor malignant potential. The invasive ability in the P-gp-positive S2/TXT cell line was not different from its P-gp-negative parental cell line SUIT-2, suggesting that TXT has no direct effect on increasing tumor malignant potential related to the induction of P-gp expression in this PAC cell line. The higher malignant potential associated with positive P-gp expression found in the other studies might be due to other invasive or metastatic related gene expressed simultaneously with P-gp.
From this study, we conclude that P-gp is primarily responsible for TXT resistance in PAC cell lines SUIT-2. However, expression of P-gp does not confer a more malignant invasive potential in this cell line with TXT resistance. Furthermore, TXT can inhibit the invasive ability of drug-sensitive cells but not drug-resistant cells.