Published online Dec 15, 2000. doi: 10.3748/wjg.v6.i6.842
Revised: July 19, 2000
Accepted: July 26, 2000
Published online: December 15, 2000
AIM: To identify the type localization and morphology of APUD endocrine cells in the gastroenteropancreatic (GEP) system of stomach-containing teleosts, and study APUD endocrine system in the stomach, intestine and pancreas of fish species.
METHODS: Two kinds of immunocytochemical (ICC) techniques of the streptavidin biotin-peroxidase complex (SABC) and streptavidin-peroxidase (S-P) method were used. The identification, localization and morphology of APUD endocrine cells scattered in the mucosa of digestive tract, intermuscular nerve plexus and glandular body of northern snakehead (Channa argus), ricefield eel (Monopterus albus), yellow catfish (Pelteobagrus ful vidraco), mandarinfish (Siniperca chuatsi), largemouth bass (Micropterus salmoides), oriental sheatfish (Silurus asotus), freshwater pomfret (Colossoma brachypomum) and nile tilapia (Tilapia nilotica) were investigated with 8 kinds of antisera.
RESULTS: The positive reaction of 5-hydroxytryptamine (5-HT) immunoreactive endocrine (IRE) cells was found in the digestive tract and glandular body of 8 fish species in different degree. Only a few gastrin (GAS)-IRE cells were seen in C. argus, M. albus and P. fulvidraco. Glucagon (GLU)-IRE cells were not found in the digestive tract and glandular body but existed in pancreatic island of most fish species. The positive reaction of growth hormone (GH)-IRE cells was found only in pancreatic island of S. Chuatsi and S. Asotus, no positive reaction in the other 6 fish species. Somatostatin (SOM), calcitonin (CAL), neurofilament (NF) and insulin (INS)-IRE cells in the stomach, intestine and pancreas of 8 kinds of fish were different in distribution and types. The distribution of all 8 APUD cells was the most in gastrointestinal epithelium mucosa and then in digestive glands. The positive reaction of SOM- and 5-HT-IRE cells was found in intermuscular nerve plexus of intestine of P. fulvidraco and S.chuatsi. Only GH-IRE cells were densely scattered in the pancreatic islands of S. chuatsi and S. asotus, and odd distribution in the pancreas of S. asotus. SOM-IRE cells were distributed in the pancreatic islands of S. asotus, C. Brachypomum and T. nilotica. There were INS-IRE cells in the pancreatic islands of S. chuatsi and S. asolus. Eight kinds of APUD cells had longer cell body and cytoplasmic process when they were located in the gastrointestinal epithelium, and had shorter cell body and cytoplasmic process in the gastric gland, and irregular shape in the esophagus and pancreatic island.
CONCLUSION: Eight kinds of IRE cells were identified in the GEP system of stomach-containing teleosts. These endocrine cells were scattered in gastrointestinal mucosa, intermuscular nerve plexus, gland body, pancreatic gland and islands under APUD system. CAL- and GH-IRE cells in the pancreatic islands of fishes showed functional diversity for these two hormones. Their morphological feature provides evidence of endocrine-paracrine and endocrine-exocrine acting mode. This research can morphologically prove that the GEP endocrine system of fish (the lowest vertebrate) is almost the same as of mammal and human.
- Citation: Pan QS, Fang ZP, Huang FJ. Identification, localization and morphology of APUD cells in gastroenteropancreatic system of stomach-containing teleosts. World J Gastroenterol 2000; 6(6): 842-847
- URL: https://www.wjgnet.com/1007-9327/full/v6/i6/842.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i6.842
In recent years, APUD (amine precursor uptake and decarboxylation) cells in the digestive tract attracted worldwide attention. It was a research domain developed quickly in recent years, especially gastrointestinal hormones[1-7]. Gastrointestinal tract was not only the digestive organ but also the biggest and most complex endocrine organ in animal[8]. It has been proved that gastrointestinal hormones were not only related with feeding behavior but also with nutrition of gastrointestinal tract. At present it has been applied to clinical practice[9-24]. The endocrine tumor of digestive tract could occur in gastrointestinal tract and liver or in pancreatic gland[25,26], even over ninety per cent of carcinoid occurred in gastrointestin[27-40]. What is the hormone’s effect on it So it is of special significance to study endocrine cells in digestive system. Up till now, researches about digestive tract in animal have been reported[41], but the research data about fish having close relations with human food are scarce. The distribution of APUD cells in the gut of 8 stomachless teleosts was reported[42]. In the present paper, ICC studies on APUD cells in the GEP system of stomach-containing teleosts were reported further, delving into difference of gastrointestinal hormone types, secretory way, distribution and cell’s shapes among fish, mammal and human, in order to provide basic materials for studying gastrointestinal endocrinology, gastroenterology, origin and prevention of disease in digestive system and its treatment.
Ricefield eel (M. albus), northern snakehead (C. argus), oriental sheatfish (S. asotus), mandarinfish (S. chuatsi), and yellow catfish (P. ful vidraco) were bought separately from the aquatic products market in Wuhan; freshwater pomfret (C. brachypomum) and nile tilapia (T. nilotica) were bought from The Second Fish and Stock Farm in Hongshan District of Wuhan; largemouth bass (M. Salmoides) was bought from Nanhu Fish Farm. We reared above 8 kinds of fish temporarily in fresh water for 24 h, then according to the methods in references[43-46], and collected the samples and made the sections. Monoclonal antibody of GH was used only in SABC ICC stain method[46], S-P ICC stain method was used in the other 7 kinds of antibody[43].
The details of the different antisera, the working dilutions and main regents used in this study are listed in Table 1.
| Antisera & regents | Working dilution | Specificity | Source |
| Human CAL | 1:400 | ZYMED Lab. Inc., USA | |
| Grass carp GH | 1:600 | Yangtze River Fisheries Institute, | |
| Chinese Academy of Fishery Sciences | |||
| Human NF | 1:400 | ZYMED Lab. Inc., USA | |
| Human INS | 1:1000 | ZYMED Lab. Inc., USA | |
| Synthetic human GAS | 1:5000 | No cross reaction with Cholecystokinin-8 | Dr. N Yanaihara & Shizuoka |
| Procine GLU | 1:1000 | Wholly cross react with | Amersham International pl. |
| Pancreatic & intestinal glucagon | |||
| Synthetic human SOM | 1:3000 | Dr. S Ito Niigata | |
| 5-HT | 1:10000 | Immunonuclear Corp., Stillwater | |
| S-P Kit | 1:100 | ZYMED Lab. Inc., USA | |
| SABC Kit (mouse IgG) | 1:100 | Boster Biotechnology Co. | |
| SABC Kit (rabbit IgG) | 1:100 | LTD., Wuhan | |
| DAB | 1:2000 | Dr.Kinji INOUE |
The steps of S-P ICC stain and control references and the SABC ICC staining steps and control followed references[43,46].
Five fishes used for each species in all 8 kinds of teleost studied, 7 specimens of each fish were observed and photomicrographed under the Olympus (BH-2) photomicroscope. The dark brown positive cells on section were counted under 10 × 20 times field. The average number of positive cells from 10 fields selected randomly in each specimen part was the IRE cell number of this part in each fish. The average number of 5 fishes of each species was quantified IRE cell’s distribution density in every part. The distribution density was showed with five grades.
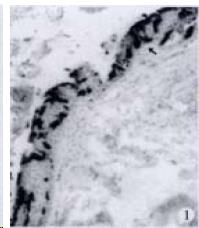
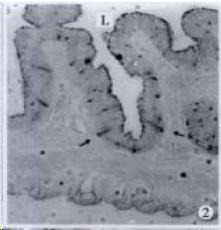
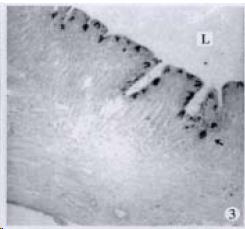
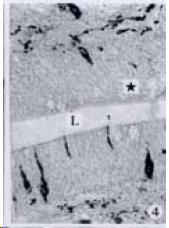
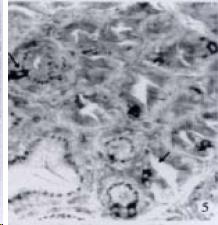
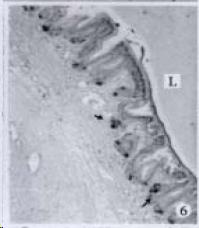
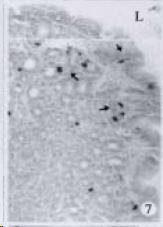
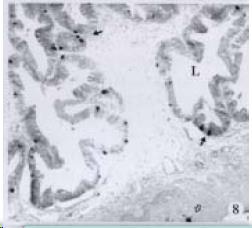
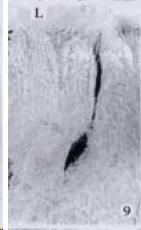
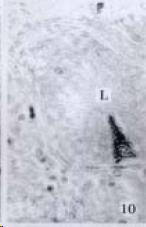
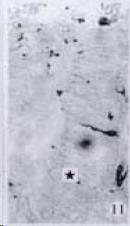
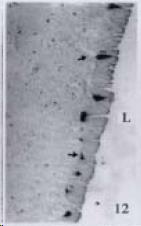
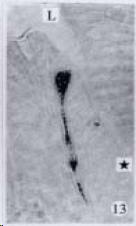
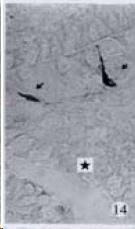
APUD cell types, distribution and density in different parts of GEP system of 8 kinds of stomach-containing teleosts are listed in Table 2. It can be seen from Table 2 that IRE cell types and distribution quantity were the least in the esophageal epithelium, only GAS, SOM and CAL-IRE cells were found in the esophageal epithelium mucosae of M. albus (Figure 1). IRE cells were the most common types and the highest distribution density in the gastric epithelium and glands of 8 kinds of fish (Figure 3, Figure 5, Figure 7, Figure 9, Figure 10, Figure 12); then in the intestine (Figure 2, Figure 4, Figure 8, Figure 11, Figure 13, Figure 14). GLU- and INS -IRE cells were mostly located in every specimen of pancreatic island. SOM- and 5-HT-IRE cells were found in the gastrointestinal intermuscular nerve plexus of P. fulvidraco and S. chuatsi separately (Figure 8, Table 2). No GAS, 5-HT, CAL- and NF-IRE cells were seen in the pancreatic island of all 8 kinds of teleosts. There were 5-HT-IRE cells in the digestive tract of all fish species; only a few GAS-IRE cells were distributed in the esophagus and stomach of C. argus, M. albus and P. fulvidraco; GH-IRE cells were distributed in a small amount in the pancreas of S. asolus, but were scattered in the pancreatic islands of S.chuatsi and S. asotus; and were not seen in the gastrointestinal tract of 8 kinds of fish species. In gastrointestinal tract, APUD cells were distributed between epithelium mucosa and glandular epithelium (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14). Negative reaction was found in all controls.
| Fish species | GAS | SOM | 5-HT | GLU | CAL | GH | NF | INS |
| Northern | 3 +++/+ | 5 +++/ | ND | 2 +/ | 1-6 ND | 1-6ND | ||
| snakehead | 4 +/ | 3 ++++/ | ||||||
| (C.argus) | 4 +++/ | |||||||
| Ricefield eel | 1 +++/+ | 1 +++/+ | 4 +++/ | ND | 1 ++++/ | 4/++ | 4/+ | |
| (M. albus) | 3 +++/++ | 3 +/ | ||||||
| 4 +/ | ||||||||
| Yellow catfish | 3/++ | 3/++++ | 3 ++/ | 7 +++/ | 1 +/ | 3 ++++/ | 2/++ | |
| (P. fulvidraco) | 4/++++ | 5 ++/ | 2 +/ | 4/+ | ||||
| 5/++ | 3 ++/ | |||||||
| 4 +++/ | ||||||||
| 5 +/ | ||||||||
| Mandarinfish | 3 +++/++ | 7 +++/ | 1-6 ND | 1-6 ND | ||||
| (S. chuatsi) | 2 +++/++ | 5 ++/ | 7 +++/ | 7 +++/ | ||||
| 4 ++/++ | 6 ++ | |||||||
| Largemouth | 3 +/ | 7 ++++/ | 7 +++/ | 1-6 ND | 1-6 ND | |||
| Bass | 4 +/ | |||||||
| (M.salmoides) | 5 +++/ | |||||||
| Oriental | 7 +++/ | 3 ++/ | 7 ++++/ | 2 +/ | 7 ++++/+ | 1-6ND | 1-6ND | |
| Sheatfish | 4 ++/ | 3/++ | 7 ++/ | |||||
| (S. asotus) | 5 ++++/ | 4/++ | ||||||
| 5 +++/ | ||||||||
| 7 +++/ | ||||||||
| Freshwater | 4 +/ | 2 +/+ | 7 ++++/ | 1 +/ | 1-7ND | 1-7ND | ||
| Pomfret | 7 ++/ | 3 ++/+ | ||||||
| (C. brachypomum) | 4 +++/+ | |||||||
| 5 +/ | ||||||||
| Nile tilapia | 3 +++/ | 2 +++/+ | 7 ++++/ | 1-7ND | 1-7ND | |||
| (T. nilotica) | 4 ++/ | 3 ++/+ | ||||||
| 5 +/ | 4 ++/+ | |||||||
| 7 ++/ | 5 +/ |
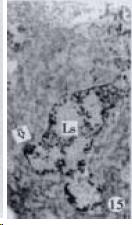
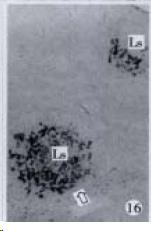
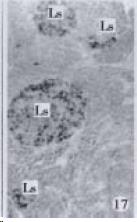
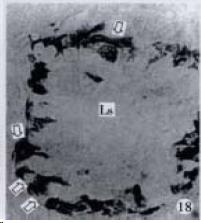
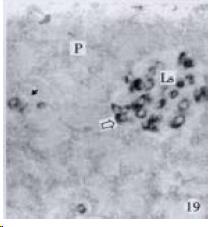
According to the distribution of APUD cells, their morphology was of great diversity. APUD cells located in stratified squamous epithelium of esophagus were scattered or piled in distribution, and irregular in shape, and had a shorter cytoplasmic process (Figure 1). While the APUD cells distributed between gastrointestinal columnar epithelium had a longer cell body, an apical cytoplasmic process extended to the gastrointestinal lumen or a basal process extended to the basement membrane (Figure 2, Figure 4, Figure 9, Figure 11, Figure 12, Figure 13, Figure 14). The APUD cells located in gastric glands were mainly pyramid-shaped (Figure 5, Figure 6, Figure 7, Figure 10), their cytoplasmic process extended to the gastrointestinal lumen. In the pancreatic islands of S. chuatsi and S.asotus, GLU-IRE cells were located in the edge of pancreatic islands (Figure 15, Figure 18) and SOM, INS- and GH-IRE cells scattered in the whole pancreatic islands (Figure 16, Figure 17, Figure 19). Their shapes were irregular, some had a longer cytoplasmic process (Figure 18), the secretory granules were clear (Figure 19) in some of IRE cells. There were a few scattered GH-IRE cells in the pancreatic exocrine area of S. asotus (Figure 19).
The distribution of APUD cells in the GEP system of stomach-containing teleosts and in the digestive system of human and stomachless teleosts is quite different. GAS-IRE cells existed in human GEP system[8,14-16] and in the intestine of 7 kinds of stomachless teleosts[42], but at present study, GAS-IRE cells were not seen in the digestive system of 7 fish species except in the esophagus of M. albus and in the stomach of C. argus and P. fulvidraco. After ICC identification of APUD cells in the gut of 7 stomachless teleosts, the 5-HT-IRE cells were not found in the gut[42]; but in the intestine of 7 stomach-containing teleosts except M. albus. Rombout and Reinecke[47] found SOM-IRE cells only existing in the stomach of stomach-containing teleosts; but at present studies, SOM-IRE cells were seen in the esophagus of M. albus, the gastric epithelium and intermuscular nerve plexus of P. fulvidraco, the gastric epithelium of S. chuatsi and T. nilotica. GLU-IRE cells existed universally in the gut of stomachless teleosts[42]; but in this study they were not found in the gastrointestinal tract of 8 stomach-containing teleosts.
It was reported that there were 4-APUD cell types: B cell (secreting INS), PP cell (secreting pancreatic polypeptide), A cell (secreting GLU) and D cell (secreting SOM) in the pancreatic island of fish species[48]. In our studies, CAL-IRE cells were found in the pancreatic island of S. asotus and M. salmoides. Calcitonin was a hormone secreted by ultimobranchial gland of fish species[49], and also was found in the secretory cells of prolactin hormone of rat hypophysis[50]. But up to now, no report showed that CAL-IRE cells exist in the pancreatic island of fish species. CAL-IRE cells in the pancreatic island of S. asotus and M. salmoides were morphologically long shuttle-shaped which was different from other endocrine cells in the pancreatic island which had the shorter cytoplasmic process and irregular shape. This specific morphological feature provides evidences that CAL-IRE cells in the pancreatic island of S. asotus and M. salmoides may adapt to some specific physiological functions and conduct endocrine paracrine action[8].
It is a common knowledge that there are GH-IRE cells in the meso-adenohypophysis of fish species and can promote the growth of them. Our studies demonstrated that there was no GH-IRE cell in the gastrointestinal tract of fish species[46], and GH was not gastrointestinal hormone. However our studies found for the first time that GH-IRE cells in the pancreatic islands of S. chuatsi and S. asotus, and in the exocrine pancreas of S. asotus had scattering distribution. It is evidenced that GH-IRE cells were not only located in the endocrine organ-pancreatic island but in the digestive gland in a small amount. It shows morphological evidence of brain-gutpeptide with an endocrine-exocrine mode of action[51]. It suggests that the GH-IRE cells existed simultaneously in the hypophysis, pancreatic island and pancreas, and the other brain-gut peptide or brain-pancreas peptide varied in pattern of distribution, and the diversity of physiological function of GH-IRE cells based on their morphology.
Edited by You DY and Ma JY
| 1. | Zhang QX, Dou YL, Shi XY, Ding Y. Expression of somatostatin mRNA in various differentiated types of gastric carcinoma. World J Gastroenterol. 1998;4:48-51. [PubMed] |
| 2. | Huang XQ. Somatostatin-probably the most widely effective gastrointestinal hormone in human body. China Natl J New Gastroenterol. 1997;3:201-204. |
| 3. | Zhu JZ, Chen DF, Leng ER. The action of a gastrointestinal hormones on regulation of gastrointestinal motion. Shijie Huaren Xiaohua Zazhi. 1999;7:687-688. |
| 4. | Yan JC, Chen WB, Liu JH, Ma Y, Xu CJ. Immunohistochemical studies on intrahepatic vascular proliferation in hepatitis B. Huaren Xiaohua Zazhi. 1998;6:780-782. |
| 5. | Wu ZJ, Xu CF, Huang YX. An immunohistochemical study on morphology and distribution of somatostotin-like immunoreactive cells in mamalian gastrointestinal tract. Xin Xiaohuabingxue Zazhi. 1997;5:654-655. |
| 6. | Zhang C, Li DG. 5-Hydroxytryptamine and the regulation of gastrointestinal tract motility. Xin Xiaohuabingxue Zazhi. 1997;5:730-731. |
| 7. | Shi AR, Liang WM, Huang Y. Localization and expression of islet amyloid polypeptide in gastrointestinal tract. Shijie Huaren Xiaohua Zazhi. 2000;8:211-213. |
| 8. | Wang ZJ, Mei MH, Zhu WY. Gastrointestinal hormones. Beijing: Science Publishing House 1985; 2-372. |
| 9. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] |
| 10. | Yan JP, Jia JB, Ma XH, Wu XR, Zhao YC, Han DW. Immunohistochemical study on expression of epidermal growth factorreceptor at hepatocyte nuclei in experimental rat liver cirrhosis. World J Gastroenterol. 1998;4:143. |
| 11. | Yu JY, Wang JL, Yao L, Zheng JY, Hu M. The changes of antral endocrine cells in Helicobacter pylori infection. China Natl J New Gastroenterol. 1995;1:25-26. |
| 12. | Li QP, Xu JQ, Xing ZH, Hu CP. Significance of gut hormones in the pathogenesis of gastric mucosal lesion in liver cirrhosis. Huaren Xiaohua Zazhi. 1998;6:789-790. |
| 13. | Yu YS, Hou YY, Zhang RY, Zhang BY, Zhang QG, Pan PE. Immunohistochemical study of ICAM_1 on liver in patients withhepatitis B. Xin Xiaohuabingxue Zazhi. 1996;4:30-32. |
| 14. | Xu CT, Yin QF, Li L, Pan BR. Serum levels of gastrin, motilin and leu_enkephalin in patients with liver cirrhosis. Xin Xiaohuabingxue Zazhi. 1996;4:25-27. |
| 15. | Liu YQ, Li DX, Cui GL, Tang FA, Li ZF. Immunohistochemical study of gastrinimmunoreactive cells in benign and malignant biospy tissues of stomach. Xin Xiaohuabingxue Zazhi. 1995;3:72-73. |
| 16. | Liang PX, Yang ZX. The alterations of gastrin, glucagon and somatostatin in patients with upper gastrointestinal haemorrhage. Xin Xiaohuabingxue Zazhi. 1997;5:172-173. |
| 17. | Jin XQ, Wu F, Lei PY, Xu JL, Chen ZY. The role of hypergastrinemia in the pathogenesis of intussusception in infants. Xin Xiaohuabingxue Zazhi. 1997;5:207-208. |
| 18. | Yan JP, Liu JC, Ma XH, Jia JB, Zhao YC, Xu RL, Li CM, Han DW. Immunohistochemical study on basic fibroblast growth factor in experimental liver fibrosis. Xin Xiaohuabingxue Zazhi. 1997;5:642-644. |
| 19. | Xu CT, Wang Y, Pan BR. Brain gut peptides in the sera and gastric juice of patients with chronic atrophic gastritis. Xin Xiaohuabingxue Zazhi. 1997;5:713-714. |
| 20. | Gao HJ, Lu XZ, Zhang XY, Zhao ZQ. AgNOR and rasp21 expression in gastric mucosal lesions with Helicobacter pyloriinfection. Xin Xiaohuabingxue Zazhi. 1997;5:715-716. |
| 21. | Chen J, Li JM, Li XH, Hao HS, Fu SH. Gastric emptying and plasma levels of gastrointestinal hormones in patients withpeptic ulcer. Xin Xiaohuabingxue Zazhi. 1997;5:717-718. |
| 22. | Yao YL, Zhang WD, Song YG. Relationship between Spleen deficiency and gastrointestinal hormones. Xin Xiaohuabingxue Zazhi. 1997;5:728-729. |
| 23. | Yan JC, Ma Y, Chen WB, Sun XH, Pei B. Immunohistochemical and electron microscopic observation on sinusoidal lesions in hepatitis B. Shijie Huaren Xiaohua Zazhi. 1999;7:943-947. |
| 24. | Wang CD, Mo JZ, Xiao SD. Changes of gastric emptying and gut hormones in patients with active duodenal ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:948-950. |
| 25. | Zhang LF, Peng WW, Yao JL, Tang YH. Immunohistochemical detection of HCV infection in patients with hepatocellular carcinoma and other liver diseases. World J Gastroenterol. 1998;4:64-65. [PubMed] |
| 26. | Zhang J, Wang WL, Li Q, Qiao Q. Expression and significance of transforming growth factor α and its receptor in human primary hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:939-942. |
| 27. | Wang DC, Wang LD, Jia YY, Liu YQ, Feng CW, Tang FA, Zhou Q, Li ZF, Cui GL. Immunohistochemical study on endocrine-like tumor cells in colorectal carcinomas. China Natl J New Gastroenterol. 1997;3:176. |
| 28. | Wang LP, Yu JY, Shi JQ, Liang YJ. Clinico-pathologic significance of neuroendocrine cells in gastric cancer tissue. China Natl J New Gastroenterol. 1996;2:30-33. |
| 29. | Wang CD, Chen YL, Wu T, Liu YR. Association between lowe expression of somatostatin receptor II gene and lymphoidmetastasis in patients with gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:864-866. |
| 30. | Wang LP, Yu JY, Deng YJ, Tian YW, Wu X, Liu G, Ding HY. Relationship between the expression of somatostatin andepidermal growth factor receptor in gastric carcinoma. Huaren Xiaohua Zazhi. 1998;6:606-609. |
| 31. | He YJ, He SW, Xie B. Clinical significance of measurement of gastrin levels in patients with colorectal carcinoma. Huaren Xiaohua Zazhi. 1998;6:707-709. |
| 32. | Xu CT, Yan XJ, Wang Y, Zhang ZC, Pan BR. Alterations of the peptides of sera and gastric juices in patients with esophageal cancer. Huaren Xiaohua Zazhi. 1998;6:513-515. |
| 33. | Xie Y, Wang CW, Zhu JQ, Zhang KH. Epidermal growth factor and somatostatin contents in plasma and tumor tissues of gastric and esophageal cancer patients. Xin Xiaohuabingxue Zazhi. 1997;5:771-772. |
| 34. | Luo ZB, Luo YH, Lu R, Jin HY, Zhang BP, Xu CP. Immunohistochemical study on dendritic cells in gastric mucosa of patients with gastric cancer and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:400-402. |
| 35. | Li QP, Yan JH. Alterations of gut hormones in gastric mucosa in patients with gastric cancer and peptic ulcer. Xin Xiaohuabingxue Zazhi. 1997;5:166-167. |
| 36. | Yang RS, Liu Q, Zhang LM, Zhang BJ, Zhang XG. Immunological modulation of cryotherapy in patients with esophageal and cardiac carcinoma. Xin Xiaohuabingxue Zazhi. 1997;5:168-169. |
| 37. | Zhang GQ, Fu LN. Diagnostic value of serum gastrin in patients with colorectal neoplasms. Xin Xiaohuabingxue Zazhi. 1997;5:178-179. |
| 38. | Quan X, Luo HS. Blood group H antigen and gastrointestinal newplasma. Xin Xiaohuabingxue Zazhi. 1997;5:185-186. |
| 39. | Wang DC, Liu YQ, Wang LD. Endocrine like tumor cells in adenocarcinoma of digestive tract. Xin Xiaohuabingxue Zazhi. 1997;5:187-188. |
| 40. | Li QM, Liu Y, Xiong HQ, Wu QM, Zou GH. Immunohistochemical study of nuclear matrix antibody in human esophageal cancer. Xin Xiaohuabingxue Zazhi. 1997;5:562-563. |
| 41. | Pan QS, Fang ZP. Present progress in the study of the APUD cells in gastro-entero pancreatic endocrine system of the fishes. Shuisheng Shengwu Xuebao. 1995;19:275-282. |
| 42. | Pan QS, Fang ZP, Zhao YX. Immunocytochemical identification and localization of APUD cells in the gut of seven stomachless teleost fishes. World J Gastroenterol. 2000;6:96-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Fang ZP, Pan QS, Nie XY, Zhao YX, Bai XM. Localization and morphology studies on calcitonin immunoreactive cells in the digestive tracts of eight species of stomach containing teleosts. Shuisheng Shengwu Xuebao. 1997;21:359-36. |
| 44. | Fang ZP, Pan QS. Identification and localization of immunoreactive endocrine cells in the pancreas of four species of sto mach containing teleosts. Shuisheng Shengwu Xuebao. 1998;22:45-48. |
| 45. | Fang ZP, Pan QS, Zhao YX. Localization and comparison of endocrine cells in the digestive tract mucosa of large mouth bass, northern snakehead and oriental sheatfish. Beijing: Chinese Forestry Publishing House 1999; 1031-1035. |
| 46. | Fang ZP, Pan QS, Chen SL, Zhao YX. Immunocytochemical localization of nine teleosts using monoclonal antibody of grass carp growth hormone. Shuisheng Shengwu Xuebao. 1998;22:355-360. |
| 47. | Rombout JH, Reinecke M. Immunohistochemical localization of (neuro)peptide hormones in endocrine cells and nerves of the gut of a stomachless teleost fish, Barbus conchonius (Cyprinidae). Cell Tissue Res. 1984;237:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Rombout JHWM. Function and origin of endocrine cells in gut and pancreas of teleosts. Acta Micros. 1985;8:329-335. |
| 49. | Shi QF. Fish Physiology. Taibei: Acquatic Products Publishing House 1994; 240-302. |
| 50. | Chen X. Calcitonin in the cells of prolactin. Shengli Kexue Jinzhan. 1990;21:30. |
| 51. | Zhu WY. Advances of brain-gut peptide studies. Shengli Kexue Jinzhan. 1982;13:15-19. |