Published online Dec 15, 2000. doi: 10.3748/wjg.v6.i6.819
Revised: September 12, 2000
Accepted: September 19, 2000
Published online: December 15, 2000
AIM: To study the molecular mechanisms of retinoic acid (RA) on proliferation and expression of cyclin-dependent kinase inhibitors (CKI), i.e. p16, p21 and p27 in cultured rat hepatic stellate cells (HSC) stimulated with transforming growth factor beta 1 (TGF-β1).
METHODS: HSC were isolated from healthy rat livers and cultured. After stimulated with 1 mg/L TGF-β1, subcultured HSC were treated with or without 1 nmol/L RA. MTT assay, immunocytochemistry (ICC) for p16, p21, p27 and α-smooth muscle actin (α -SMA) protein, in situ hybridization (ISH) for retinoic acid receptor beta 2 (RAR-β2) and p16, p21 and p27 mRNA and quantitative image analysis (partially) were performed.
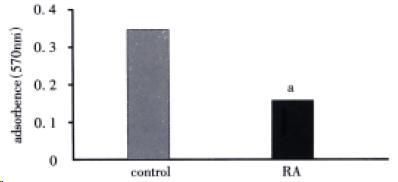
RESULTS: RA inhibited HSC proliferation (41.50%, P < 0.05), decreased the protein level of α-SMA (55.09%, P < 0.05), and induced HSC to express RAR-β2 mRNA. In addition, RA increased the protein level of p16 (218.75%, P < 0.05) and induced p21 protein expression; meanwhile, p27 was undetectable by ICC in both control and RA-treated HSC. However, RA had no influence on the mRNA levels of p16, p21 or p27 as determined by ISH.
CONCLUSION: Up-regulation of p16 and p21 on post-transcriptional level may contribute, in part, to RA inhibition of TGF-β1 initiated rat HSC activation in vitro.
- Citation: Huang GC, Zhang JS, Zhang YE. Effects of retinoic acid on proliferation, phenotype and expression of cyclin-dependent kinase inhibitors in TGF-β1-stimulated rat hepatic stellate cells. World J Gastroenterol 2000; 6(6): 819-823
- URL: https://www.wjgnet.com/1007-9327/full/v6/i6/819.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i6.819
Hepatic stellate cells (HSC) play crucial roles in the development of liver fibrosis[1-6]. Stimulated HSC transform from vitamin A-rich quiescent cells to myofibroblast-like cells characterized by the expression of α-smooth muscle actin (α-SMA), loss of retinoids and diminished retinoid signaling[4-15]. Exogenous retinoids such as retinoic acid (RA) may recover the contents of retinoids and nuclear retinoic acid receptors (RAR) in HSC and therefore suppress hepatic fibrogenesis, but the mechanisms of RA on HSC inhibition were not well understood[3,16-27]. Recent studies on other cell types have shown that modulation of cell cycle regulatory proteins might contribute to RA-induced inhibition of cell prolifer ation and differ-entiation[28-37] and Kawada et al[38] reported that expression of G1 cyclin was involved in cell cycle transition of HSC from G1 to S. The present study was designed to investigate the effects of RA on negative cell cycle regulators cyclin-dependent kinase inhibitors (CKI) in cultured rat HSC stimulated with transforming growth factor beta-1 (TGF-β1). The results showed that RA inhibited HSC activation may be in part due to post-transcriptional up-modulation of p16 and p21.
Collagenase IV, pronase E, Nycodenz, RA and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide or tetrazolium (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Recombinant human TGF-β1 was from Oncogene Science (Uniondale, NY, USA). Dulbecco’s modified Eagle’s medium (DMEM) was Gibco/BRL-product (Life Technologies, Inc. Grand Island, NY, USA). Newborn calf serum, plastic tissue culture flasks and multi-plates were from Corning Incorporated (Corning, NY, USA). Polyclone anti-α-SMA antibody was purchased from Dako A/S (Glostrup, Denmark). Antibodies to p16, p21 and p27 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). ABC kit and DAB were from R&D Systems (Minneapolis, MN, USA). DIG Nucleic Acid Label and Detect Kit and Taq DNA polymerase were from Roche Diagnostics GmbH (Mannheim, Germany).
Cells were isolated from healthy Sprague-Dawley male rats (weighing 400 g-450 g) as described by Weiner et al[26] with minor modifications by the laboratory[39], seeded onto 25 cm2 plastic tissue culture flasks and incubated at 37 °C in a humidified 5% CO2/95% air. The medium was replaced 24 h after seeding and every 48 h thereafter. After they reached confluence (10 d after planting), activated HSC were subcultured onto plastic tissue culture multi-plates with or without coverslips.
Experiments were performed on cells between serial passage 1 and 3 using three independent cell lines.
Activated HSC were depleted of serum for 48 h, followed by incubation with 1 mg/L TGF-β1 for another 48 h, and then the medium was removed and cells maintained in DMEM with or without 1 nmol/L RA for 48 h. Preliminary dose dependence experiments indicated that 1 mg/L TGF-β1 or 1 nmol/L RA had significant influence on HSC proliferation.
Cell proliferation was measured by MTT assay as previously described[40] with minor modifications. Briefly, during the last 4 h of incubation the cells were loaded with 10 μL of freshly prepared and filtered MTT (5 g/L in PBS) per well. The medium was then replaced with 100 μL absolute ethanol and the cells were left for 30 min for color development, followed by reading on Vmax® Kinetic Microplate Reader (Molecular Devices Corporation, Sunnyvale, California, USA) at 570 nm wavelength.
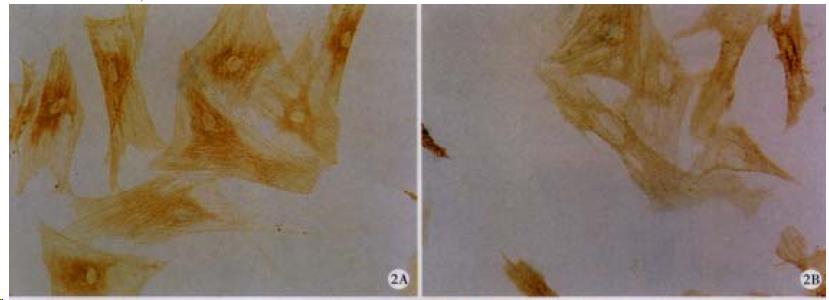
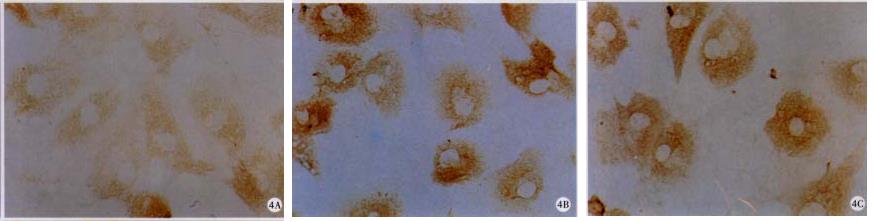
Cells grew on coverslips were fixed, permeabilized, blocked with 1% serum in PBS, and then incubated with primary antibodies to either α-SMA, p16, p21 or p27. ABC assay and DAB system were used to detect the proteins[41] and photomicrographs were taken with an Olympus microphoto-microscope (Olympus Optical Co. LTD., Shinjuku-ku, Tokyo, Japan).
cDNA probes for human RAR-β2 and p16 were gifts from the Department of Biochemistry, School of Basic Medical Sciences, Fudan University; and cDNA fragments for rat p21 and p27 were presented as gifts by Dr. Chen Guang-Ping. Fragments were labeled with digoxigenin using random priming assay.
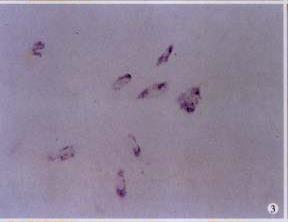
ISH was performed as previously described[39] with immunohistochemical detection using an alkaline phosphatase (AKP) conjugated anti-digoxigenin monoclonal antibody. Hybridization signal was visualized through the substrates of AKP (NBT and BCIP). Photomicrographs were taken with an Olympus microphoto-microscope again.
Quantitative analysis of protein and mRNA were performed by scanning using KS 400 Imaging System 3.0 (Carl Zeiss Vision GmbH, Germany) and means of density values were determined.
Data were presented as mean values ± S.D. and statistical significance was assessed by Student’s t test.
As shown in Figure 1, there were fewer (41.50%, P < 0.05) HSC in RA-treated cells compared with control cells. In addition, RA decreased expression of α-SMA (55.09%, P < 0.05; Figure 2 and Table 1).
To evaluate retinoid signaling, ISH was performed to determine RAR-β2 gene expression. No mRNA was detected in control cells, but HSC treated with RA did express RAR-β2, indicate RA induced expression of RAR-β2 in HSC-(Figure 3), and therefore enhanced retinoid signaling.
To further clarify the mechanisms of RA on cell cycle regulation in HSC, protein and mRNA levels of CKI were determined. As shown in Figure 4, p27 was undetectable by ICC in both control and RA-treated HSC. In addition, RA increased the protein levels of p16 (218.75%, P < 0.05) and p21 protein was detected in HSC treated with RA (Figure 4 and Table 1).
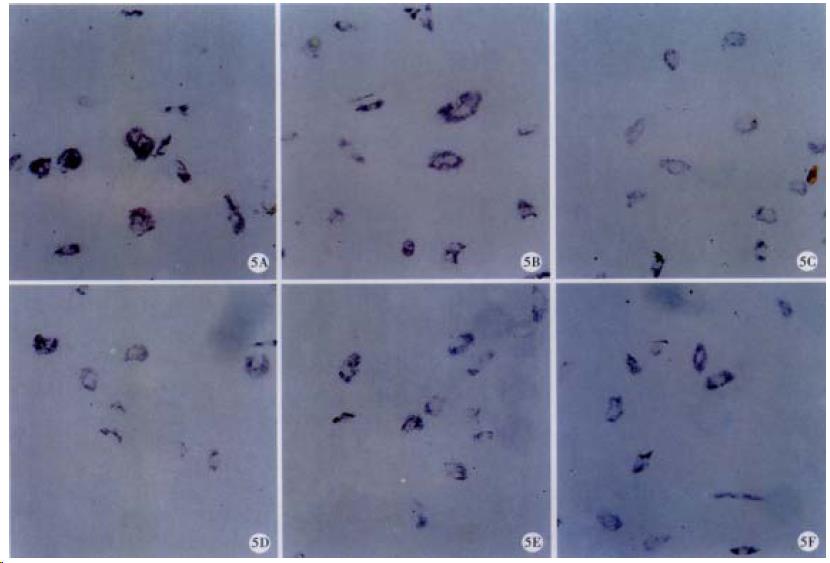
ISH results showed that the mRNA level of p16, p21 or p27 was not influenced by RA (Figure 5 and Table 1).
TGF-β1 is one of the most fibrogenetic cytokines on HSC, which initiates HSC activation characterized by loss of retinoids, proliferation, and expression of α-SMA and extracellular matrix[2,6,42-45]. Our results showed that even 48 h depletion of serum could not completely suppress the expression of α-SMA, implying that serum depletion can not reversibly suppress TGF-β1-initiated activation of rat HSC in culture.
RA may modulate cell growth and differentiation through retinoid signaling [30,46-49], mainly by nuclear retinoid X receptors and RAR. Present study showed that RA inhibited HSC proliferation and down-regulated α-SMA protein, demonstrating that RA may suppress HSC activation induced by TGF-β1. Our results showed that RA induced RAR-β2 mRNA, which may then modulate expression of some other genes including CKI[28-37,50-52]. In addition, cells in controls displayed no RAR-β2 mRNA, agreeing with its insufficient to completely suppress HSC activation again.
The protein level of p16 was increased in RA-treated HSC with detectable p21 protein, while RA had no influence on those mRNA levels, suggesting RA may up-regulate p16 and p21 gene expression on the post-transcriptional level. p16 or p21 can inhibit cyclin-CDK complexes and then prevent G1 transition[53-58]; therefore, our study indicates that RA induced inhibition of TGF-β1-initiated HSC activation may be in part due to up-modulation of p16 and p21 on the post-transcriptional level, and reveals a new mechanism of RA induced HSC inhibition.
Edited by You DY
Proofread by Ma JY
| 1. | Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 390] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Senoo H, Imai K, Matano Y, Sato M. Molecular mechanisms in the reversible regulation of morphology, proliferation and collagen metabolism in hepatic stellate cells by the three-dimensional structure of the extracellular matrix. J Gastroenterol Hepatol. 1998;13 Suppl:S19-S32. [PubMed] |
| 4. | Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836-847. [PubMed] |
| 6. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Wang XD. Chronic alcohol intake interferes with retinoid metabolism and signaling. Nutr Rev. 1999;57:51-59. [PubMed] |
| 8. | Ohata M, Lin M, Satre M, Tsukamoto H. Diminished retinoic acid signaling in hepatic stellate cells in cholestatic liver fibrosis. Am J Physiol. 1997;272:G589-G596. [PubMed] |
| 9. | Huang GC. Retinoid signaling in liver and hepatic fibrosis. Guowai Yixue Shengli Bingli Kexue Yu Linchuang Fence. 2000;20:109-111. |
| 10. | Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271-277. [PubMed] |
| 11. | Pinzani M. Novel insights into the biology and physiology of the Ito cell. Pharmacol Ther. 1995;66:387-412. [PubMed] |
| 12. | Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39-S45. [PubMed] |
| 13. | Friedman SL. The cellular basis of hepatic fibrosis. New Engl J Med. 1993;24:1828-1835. |
| 14. | Geerts A, Lazou JM, De Bleser P, Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991;13:1193-1202. [PubMed] |
| 15. | Gressner AM. Hepatic fibrogenesis: the puzzle of interacting cells, fibrogenic cytokines, regulatory loops, and extracellular matrix molecules. Z Gastroenterol. 1992;30 Suppl 1:5-16. [PubMed] |
| 16. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (. Lond). 1997;92:103-112. [PubMed] |
| 17. | Vicente CP, Fortuna VA, Margis R, Trugo L, Borojevic R. Retinol uptake and metabolism, and cellular retinol binding protein expression in an in vitro model of hepatic stellate cells. Mol Cell Biochem. 1998;187:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Davis BH, Pratt BM, Madri JA. Retinol and extracellular collagen matrices modulate hepatic Ito cell collagen phenotype and cellular retinol binding protein levels. J Biol Chem. 1987;262:10280-10286. [PubMed] |
| 19. | Davis BH, Rapp UR, Davidson NO. Retinoic acid and transforming growth factor beta differentially inhibit platelet-derived-growth-factor-induced Ito-cell activation. Biochem J. 1991;278:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Davis BH, Vucic A. The effect of retinol on Ito cell proliferation in vitro. Hepatology. 1998;8:788-793. [PubMed] |
| 21. | Davis BH, Coll D, Beno DW. Retinoic acid suppresses the response to platelet-derived growth factor in human hepatic Ito-cell-like myofibroblasts: a post-receptor mechanism independent of raf/fos/jun/egr activation. Biochem J. 1993;294:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Davis BH, Kramer RT, Davidson NO. Retinoic acid modulates rat Ito cell proliferation, collagen, and transforming growth factor beta production. J Clin Invest. 1990;86:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Matsuura T, Hasumura S, Nagamori S, Murakami K. Retinol esterification activity contributes to retinol transport in stellate cells. Cell Struct Funct. 1999;24:111-116. [PubMed] |
| 24. | Seifert WF, Bosma A, Hendriks HF, van Leeuwen RE, van Thiel-de Ruiter GC, Seifert-Bock I, Knook DL, Brouwer A. Beta-carotene (provitamin A) decreases the severity of CCl4-induced hepatic inflammation and fibrosis in rats. Liver. 1995;15:1-8. [PubMed] |
| 25. | Sato T, Kato R, Tyson CA. Regulation of differentiated phenotype of rat hepatic lipocytes by retinoids in primary culture. Exp Cell Res. 1995;217:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Weiner FR, Blaner WS, Czaja MJ, Shah A, Geerts A. Ito cell expression of a nuclear retinoic acid receptor. Hepatology. 1992;15:336-342. [PubMed] |
| 27. | Gao ZL, Li DG, Lu HM, Gu XH. The effect of retinoic acid on Ito cell proliferation and content of DNA and RNA. World J Gastroenterol. 1999;5:443-444. [PubMed] |
| 28. | Bocchia M, Xu Q, Wesley U, Xu Y, Korontsvit T, Loganzo F, Albino AP, Scheinberg DA. Modulation of p53, WAF1/p21 and BCL-2 expression during retinoic acid-induced differentiation of NB4 promyelocytic cells. Leuk Res. 1997;21:439-447. [PubMed] |
| 29. | Pomponi F, Cariati R, Zancai P, De Paoli P, Rizzo S, Tedeschi RM, Pivetta B, De Vita S, Boiocchi M, Dolcetti R. Retinoids irreversibly inhibit in vitro growth of Epstein-Barr virus-immortalized B lymphocytes. Blood. 1996;88:3147-3159. [PubMed] |
| 30. | Liu M, Iavarone A, Freedman LP. Transcriptional activation of the human p21 (WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J Biol Chem. 1996;271:31723-31728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 247] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Jeong JS, Lee JH, Kim HI, Park JI. Changes in expression of cell cycle regulators and their hepatic lobular distribution in partial hepatectomy-induced regenerating rat liver. J Korean Med Sci. 1999;14:635-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Jaumot M, Estanyol JM, Serratosa J, Agell N, Bachs O. Activation of cdk4 and cdk2 during rat liver regeneration is associated with intranuclear rearrangements of cyclin-cdk complexes. Hepatology. 1999;29:385-395. [PubMed] |
| 33. | Gill RM, Slack R, Kiess M, Hamel PA. Regulation of expression and activity of distinct pRB, E2F, D-type cyclin, and CKI family members during terminal differentiation of P19 cells. Exp Cell Res. 1998;244:157-170. [PubMed] |
| 34. | Dirks PB, Patel K, Hubbard SL, Ackerley C, Hamel PA, Rutka JT. Retinoic acid and the cyclin dependent kinase inhibitors synergistically alter proliferation and morphology of U343 astrocytoma cells. Oncogene. 1997;15:2037-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Zhu WY, Jones CS, Kiss A, Matsukuma K, Amin S, De Luca LM. Retinoic acid inhibition of cell cycle progression in MCF-7 human breast cancer cells. Exp Cell Res. 1997;234:293-299. [PubMed] |
| 36. | Matsuo T, Thiele CJ. p27Kip1: a key mediator of retinoic acid induced growth arrest in the SMS-KCNR human neuroblastoma cell line. Oncogene. 1998;16:3337-3343. [PubMed] |
| 37. | Hsu SL, Chen MC, Chou YH, Hwang GY, Yin SC. Induction of p21 (CIP1/Waf1) and activation of p34 (cdc2) involved in retinoic acid-induced apoptosis in human hepatoma Hep3B cells. Exp Cell Res. 1999;248:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Kawada N, Ikeda K, Seki S, Kuroki T. Expression of cyclins D1, D2 and E correlates with proliferation of rat stellate cells in culture. J Hepatol. 1999;30:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Li WC, Zhang JS, Huang GC, Zhu HG, Zhang XR, ZhangYE . Effects of heparin on the growth, extracellular matrix and matrix metalloproteinase gene expression in rat hepatic stellate cells. Zhonghua Ganzangbing Zazhi. 2000;8:200-202. |
| 40. | Hussain RF, Nouri AM, Oliver RT. A new approach for measurement of cytotoxicity using colorimetric assay. J Immunol Methods. 1993;160:89-96. [PubMed] |
| 41. | Chen Y, Zhao Z, Gu Y, Huang G. [Immunohistochemical study on myoglobin in electrocution]. Fa Yi Xue Za Zhi. 1997;13:12-3, 64. [PubMed] |
| 42. | De Bleser PJ, Niki T, Rogiers V, Geerts A. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol. 1997;26:886-893. [PubMed] |
| 43. | Bedossa P, Paradis V. Transforming growth factor-beta (TGF-beta): a key-role in liver fibrogenesis. J Hepatol. 1995;22:37-42. [PubMed] |
| 44. | Bachem MG, Meyer D, Schäfer W, Riess U, Melchior R, Sell KM, Gressner AM. The response of rat liver perisinusoidal lipocytes to polypeptide growth regulator changes with their transdifferentiation into myofibroblast-like cells in culture. J Hepatol. 1993;18:40-52. [PubMed] |
| 45. | Liu F, Liu JX. Role of transforming growth factor β1 in the liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 2000;8:86-88. |
| 46. | Senoo H, Wake K. Suppression of experimental hepatic fibrosis by administration of vitamin A. Lab Invest. 1985;52:182-194. [PubMed] |
| 47. | McCormack SA, Viar MJ, Tague L, Johnson LR. Altered distribution of the nuclear receptor RAR beta accompanies proliferation and differentiation changes caused by retinoic acid in Caco-2 cells. In vitro Cell Dev Biol Anim. 1996;32:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924-2933. [PubMed] |
| 49. | Gao CF, Wang H, Kong XT. The effects of Vitamine A on 3T3 cells, liver lipocytes proliferation and procollagen III experssion. Xin Xiaohuabingxue Zazhi. 1996;4:9-11. |
| 50. | Lavelle D, Chen YH, Hankewych M, Desimone J. Inhibition of myeloma cell growth by all-trans retinoic acid is associated with upregulation of p21WAF1 and dephosphorylation of the retinoblastoma protein. Leuk Lymphoma. 1999;35:261-268. [PubMed] |
| 51. | Um SJ, Kim EJ, Hwang ES, Kim SJ, Namkoong SE, Park JS. Antiproliferative effects of retinoic acid/interferon in cervical carcinoma cell lines: cooperative growth suppression of IRF-1 and p53. Int J Cancer. 2000;85:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Borriello A, Pietra VD, Criscuolo M, Oliva A, Tonini GP, Iolascon A, Zappia V, Ragione FD. p27Kip1 accumulation is associated with retinoic-induced neuroblastoma differentiation: evidence of a decreased proteasome-dependent degradation. Oncogene. 2000;19:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Parry D, Mahony D, Wills K, Lees E. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol Cell Biol. 1999;19:1775-1783. [PubMed] |
| 54. | Gombart AF, Yang R, Campbell MJ, Berman JD, Koeffler HP. Inhibition of growth of human leukemia cell lines by retrovirally expressed wild-type p16INK4A. Leukemia. 1997;11:1673-1680. [PubMed] |
| 55. | Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2020] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 56. | Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2515] [Cited by in RCA: 2557] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 57. | Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995;147:545-560. [PubMed] |
| 58. | Moustakas A, Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci USA. 1998;95:6733-6738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 282] [Article Influence: 10.4] [Reference Citation Analysis (0)] |