INTRODUCTION
Only the liver has the great capability of regeneration in mammal[1]. Few hepatocytes are in the phase of division in the normal liver of an adult mammal (including human beings)[2-4], but the remaining hepatocytes can be induced to proliferate quickly by partial hepatectomy (PH), and, to some degree, they stop dividing and re-differentiate into cells functioning as hepatocytes[5,6]. This shows that liver regeneration is a delicate process in which the cellular dedifferentiation, multiplication and re-differentiation are regulated accurately[7-9]. In the past decades, the liver regenerati on of mammal has been regarded as one of the best model to study the restructuring and regeneration of tissues and organs, cellular multiplication, dedifferentiation, re-differentiation, stress response and regulation of physiology and biochemistry[10-13]. Most studies of liver regeneration focus on observing the changes in cellular morphology, structure, physiology, biochemistr y, metabolism[14-16], seperation and function of liver regeneration factors[17-20], and how to initiate the regeneration of hepatocytes. However, there are few studies focusing on some biomacromoleculers, e.g. heat shock proteins, proteinases, phosphatases, peroxidases in connection with the above. How these biomacromoleculers affect liver regeneration is very worth studying. This paper reports some preliminary approaches regarding these aspects.
MATERIALS AND METHODS
Materials
Sprague-Dawfey rats (Weighing 200 g-250 g) were provided by the experimental animal house of College of Life Science of Henan Normal University. Chemicals and reagents were of analytical grade, rat monoclonal antibody-1 HSC70/HSP68 (StressGen SPA-820) from anti-human HSC70/HSP68 combined specifically with 1-180 amino acids region of N-end of HSC70/HSP68 of humans, primates, rabbit, rat and ox; antibody-2 (Sino-American) was goat anti-mouse IgG-AP, S-P was marked by peroxidase (Sino-American).
Methods
Samples The rats were divided into groups at random (5 rats/group). In the first group (L): under ether anaesthesia, 2/3 of the liver was cut according to the method of Higgins[21], and rats were made to recover for 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 16 h, 20 h, 24 h, 36 h, 48 h, 72 h, 96 h and 144 h at room temperature. The second group (H-L): 2/3 of the liver was cut when recovered for 8 h at room temperature (25 °C) after heat shock (at 46 °C for 30 min), and then recovered as the first group. The third group (L-H): 2/3 of the liver was cut and recovered for 4 h before heat shock (at 46 °C for 30 min), and then recovered as the first group. The rats were killed and bled by taking off the eyeballs. Liver was washed until it became white through coronary vein perfusion[22], then put into the culture dish with icy physiological salt solution, and sheered into pieces for homogenating in the buffer (40 mmol/L NaCl, 20 mmol/L Tris-HCl, pH7.5) at 4 °C, and centrifugated for 10 min at 12000 × g. The supernatant was aliquoted and stored at -85 °C.
Spectrophotometry of the activity of ACP and AKP
Homogenate 0.1 mL was added to 0.4 mL buffer (0.25 mol/L MgCl2, 0.2 mol/L Tris-Cl, pH7.5 for AKP; 0.35 mol/L C6H7O7Na·2H2O NaOH-HCl, pH5 for ACP), and 0.4 mL 0.01 mol/L β-glycerophosphoric acid sodium (0.4 mL physiological salt solution as control), mixed and incubated for 30 min at 37 °C, added with 0.1 mL 50% C2O2Cl3 (trichloroacetic acid and homogenate were added simultaneously for control), remixed, then added 2 mL distilled water and 3 mL reagent (3 mol/L H2SO4:H2O:0.02 mol/L (NH4)6Mo7O24·4H2O:0.6 mol/L Vit C = 1:2:1:1) mixed and incubated for 25 min at 45 °C, and cooled at room temperature. The photoabsorption value at 660 nm was determined and the content of phosphate from standard curve calculated.
Qualitative analysis of HSC70/HSP68 by stereologic method
According to Weibail[23], five pieces of sections were counted at high power (5 × 10 and 5 × 40) for each sample, and the number of positive cells (Pxi) and total cell of detecting field of Vision (Pri) counted seperately. By the equation of Uv = ∑Pxi/∑pri, the stereodensity of positive liver cells was calculated, the highest value was regarded as 100%, then statistic analysis was made.
RESULTS
Change of comparative content of HSC70/HSP68 and activity of ACP and AKP in the first group
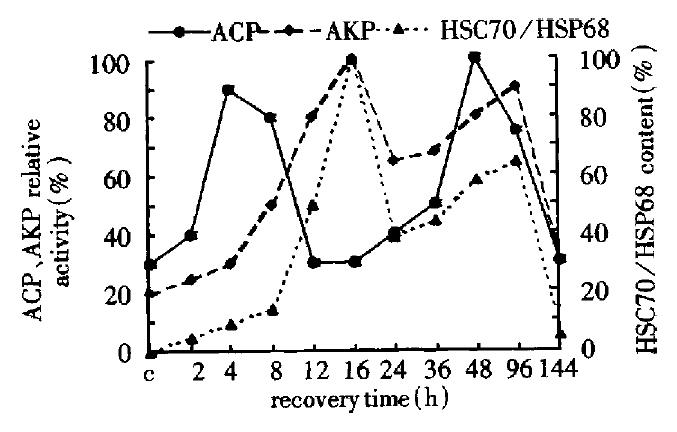
During the liver regeneration (0 h-144 h), the activity of ACP was extremely strong at 4 h and 48 h and that of AKP was at 16 h and the content of HSC70/HSP68 was at 96 h. The change of AKP activity and heat shock protein content were almost consistent, however, the peak of ACP activity occurred ahead of 12 h and 24 h as compared with the two former ones correspondingly (Figure 1).
Figure 1 Change of the HSC70/HSP68 content, ACP and AKP activity in the first group.
Change of comparative content of HSC70/HSP68 and activity of ACP and AKP in the second group
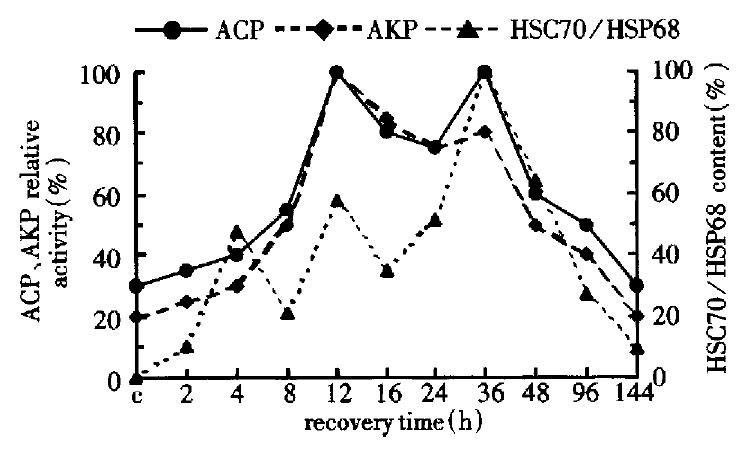
During liver regeneration after heat shock (0 h-144 h), the activity of ACP and AKP changed almost consistently, which reached the peaks at 16 h and 36 h. Although the content of HSC70/HSP68 had three peaks (4 h, 12 h and 36 h), the change was similar to that of ACP and AKP after 8 h in liver regeneration (Figure 2).
Figure 2 Change of HSC70/HSP68 the content, ACP and AKP activity in the second group.
Change of comparative content of HSC70/HSP68 and activity of ACP and AKP in the third group
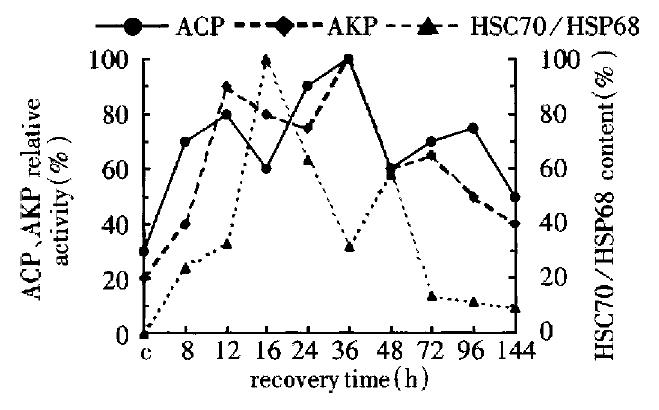
During liver regeneration (0 h-144 h), the activity of ACP was very extremely strong at 12 h and 36 h, and had the small peak at 72 h. But AKP had three peaks at 12 h, 36 h and 96 h, and that of HSC70/HSP68 content appeared at 36 h and 48 h. The change of ACP activity was similar to that of AKP, but it was negatively relative to that of HSC70/HSP68-content (Figure 3).
Figure 3 Change of the HSC70/HSP68 the content, AKP and ACP activity in the third group.
Change of comparative activity of ACP in the three groups
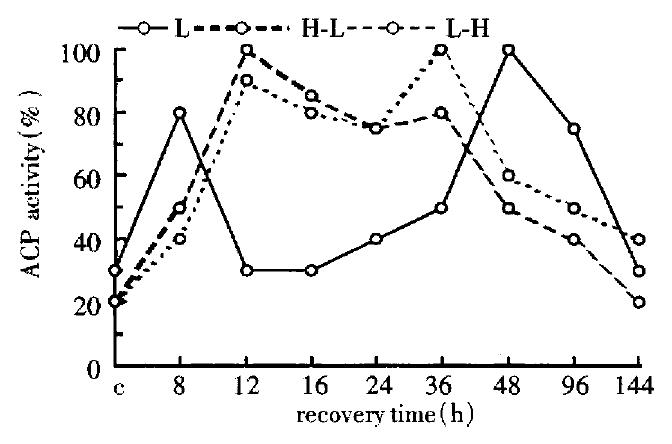
The change of ACP activity was very similar in the second group (H-L) and the third group (L-H), but being different greatly from that of the first group (Figure 4).
Figure 4 Change of the ACP activity in the three groups (by spectrophotometry).
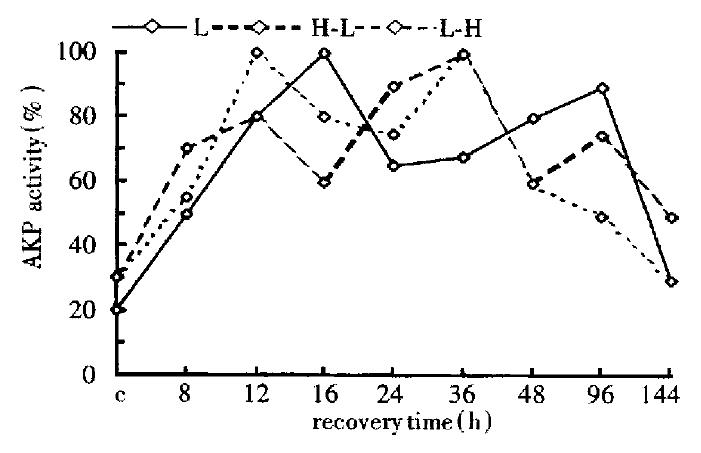
Change of comparative activity of AKP in the three groups
The change of AKP activity was very complex in the three groups, from 8 h to 12 h, AKP activity all went up; but, at 12 h-48 h, that of AKP in the first group was almost inverse to that of the other two groups; at 96 h, they were proportional in the first and the second group, and negatively relative to that of the third group (Figure 5).
Figure 5 Change of the AKP activity in the three groups (by spectrophotometry).
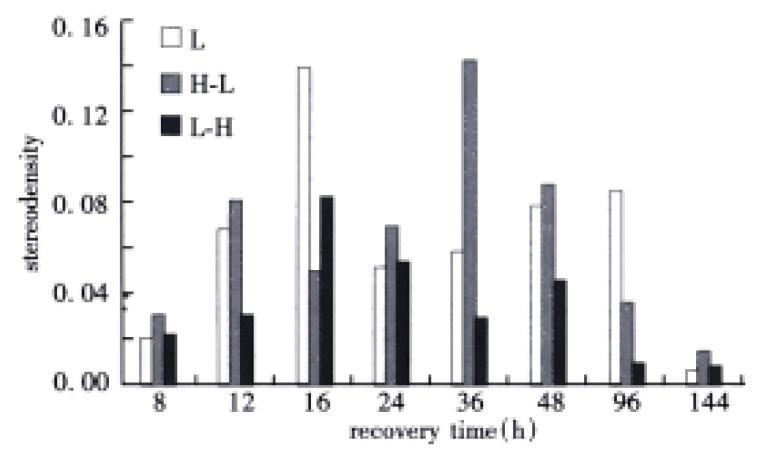
Change of comparative content of HSC70/HSP68 in the three groups
The content of HSC70/HSP68 had two peaks at 16 h and 96 h in the first group, but at 12 h and 36 h in the second group, and at 16 h and 48 h correspondingly in the third group. Moreover, the content of HSC70/HSP68 was the lowest in the third group (Figure 6).
Figure 6 Change of the HSC70/HSP68 content in the three groups (by stereologic analysis).
DISCUSSION
The reversible regulation mechanism of phosphorylation and dephosphorylation and the irreversible regulation mechanism of protein degradation are regarded as two important ways which regulate the living action of cells[24-27]. As we know, that a series of signal transductive pathways are activited and many proteins are phosphorylated and dephosphorylated are involved in promoting G0-phase cell into cell cycle[28-30]. In the early phase of liver regeneration, the increase of phosphotase activities (ACP and AKP) can be related to G0-phase cell into cell cycle. However, the peaks of ACP and AKP activity can be related to the two hepatocyte cycles (0 h-36 h and 36 h-96 h) and a non-hepatocyte cycle (96 h-114 h)[31] during the whole process of liver regeneration (0 h-144 h) .
In the first group, the first peak of ACP and AKP activity appeared at G1/S checkpoint and S-phase respectively. In the second and the third group, perhaps, because of heat shock, the peak of ACP was delayed, that of AKP was moved up, they all appeared at 12 h, but the second peak of them were also consistent. It is supposed that heat shock can affect the synthes is of normal protein (including ACP). When cell can synthesize normal protein, some cells have entered into the first S-phase during liver regeneration, at which ACP and AKP may be synthesized simultaneously. That there were three peaks of AKP activity and the second and third peak just appeared at G1-phase of liver regeneration are well worth further studying.
The family of HSP70 is known as molecular chaperone, which can help the new peptides to transfer, promote the new polypeptides to trim and to assemble in endoplasmic reticulum[32-34]. Moreover, the family of HSP73 can recognize the polypeptides with KFEKQ sequence and combine them, and help the protein in cell transfer into lysosome to degra date[35,36]. The process of liver regeneration with the synthesis and degradation of many proteins may need the family of HSP70. In recent years, the studies show that heat shock proteins play an important role in cellular differentiation, dedifferentiation and proliferation[37,38]. The first peak of heat shock protein content reported in this paper was just at 12 h-16 h during liver regeneration, at which hepatocytes are in S-phase; the second peak was just the peak of non-hepatocyte in S-phase. When rats treated by heat shock before partial hepatectomy, the first peak of HSC70/HSP68 was delayed by 20 h or so. These showed that heat shock may affect the expression of HSC70/HSP68. In contrast, treated rats by heat shock after partial hepatectomy, the peak of HSC70/HSP68 expression appeared at the same time as that of only partial hepatectomy group (at 16 h), however, the content of HSC70/HSP68 in the former group was decreased by 40% than the latter. The results were consistent with that of two continuous treatment by heat shock done by Xu et al[39].
In general, liver regeneration is a very complex physiological and physiochemical process. How on earth HSC70/HSP68, proteinase, ACP and AKP affect only and/or cooperately during liver regeneration should be further studied.