Published online Oct 15, 2000. doi: 10.3748/wjg.v6.i5.681
Revised: July 24, 2000
Accepted: July 31, 2000
Published online: October 15, 2000
AIM: To study the effect of a varying concentrations of arsenic trioxide on human hepatoma cell line BEL-7402 cultured in vitro and its mechanism of action.
METHODS: The BEL-7402 cells were treated with arsenic trioxide (at the concentrations of 0.5, 1, 2 μmol/L, respectively) for 4 successive days. The cell growth and proliferation were observed by cell counting and cell-growth curve. Morphologic changes were studied with electronmicroscopy. Flow cytometry was used to assay cell-DNA distribution and the protein expression of Bcl-2 and Bax detected by immuno cytochemical method.
RESULTS: The cell growth was significantly inhibited by varying concentrations of arsenic trioxide as revealed by cell counting and cell-growth curve, which was dose- and time-dependent. Arsenic trioxide treatment at 0.5, 1 and 2 μmol/L resulted in a sub G1 cell peak, the apoptosis rate of the control group was 9.31% and that of 0.5 μmol/L arsenic trioxide 15.53%, no significant difference was seen between the two. The apoptosis rates of 1, 2 μmol/L arsenic trioxide were 19.10% and 21.87% respectively, which were much higher (both P < 0.05). Decrease of G0/G1 phase cells and increase of S phase cells were observed by flow cytometry, suggesting the inhibition effect of 0.5, 1, 2 μmol/L arsenic trioxide on BEL-7402 cell lay in the G0/G1 phase. Morphologic changes such as intact cell membrane, nucleic condensation, apoptotic body formation were seen under transmission electronmicrescopy, whereas the 0.5 mol/L arsenic trioxide-treated BEL-7402 cells showed decrease of nucleocytoplasmic ratio, round nucleus, well-differentiated organelles in the cytoplasm. The processes and microvilli on the cell surface of the experimental groups under scanning electron microscopy were significantly decreased. High expressions of Bcl-2 and Bax were detected in 1 and 2 μmol/L arsenic trioxide-treated cells, these were 46%, 87.33% and 83.08%, 95.83% respectively, among which that of Bax was more significant. Arsenic trioxide treatment at 0.5 μmol/L resulted in a higher expression level of Bcl-2 and lower expression level of Bax, which were 8.81% and 3.83% respectively, as compared with that of the control group (15.33%) (P1 <0.01, P2 < 0.01).
CONCLUSION: Arsenic trioxide not only inhibited proliferation but also induced apoptosis of human hepatoma cell line BEL-7402. The induced-apoptosis effect of 1, 2 μmol/L arsenic trioxide was related to the expression level of Bcl-2 and Bax.
-
Citation: Xu HY, Yang YL, Gao YY, Wu QL, Gao GQ. Effect of arsenic trioxide on human hepatoma cell line BEL-7402 cultured
in vitro . World J Gastroenterol 2000; 6(5): 681-687 - URL: https://www.wjgnet.com/1007-9327/full/v6/i5/681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i5.681
Arsenic trioxide is the main ingredient of traditional Chinese medicinal, pi shi. Zhang P, et al first reported the effect of Arsenic trioxide on promyelocytic leukemia (APL) with satisfactory results. The rate of complete remission in patients who had not received any treatment before reached 73.33%, and was 52.38% in patients with recurrence, the longest remission period was more than ten years, and intravenous route of administration was the choice, no toxic or adverse effects were seen[2-4]. There was no cross resistance between arsenic trioxide and other chemical drugs during the course of treatment of APL[5]. Shen[6] concluded that arsenic trioxide treatment was effective and relatively safe in APL patients refractory to ATRA and conventional chemotherapy. Inorganic arsenic trioxide was recently shown to induce apoptosis in NB4 promyelocytic leukemic cells[7]. The present study was so designed as to broaden the anti-tumor spectrum and to study the inhibitory effect of Arsenic trioxide on human hepatoma cell line and its mechanism of action, in order to provide some theoretical basis for its clinical use.
Human hepatoma cell line BEL-7402 was purchased from the Cell Institute of Chinese Academy of Science. Arsenic trioxide was produced in Pharmaceutical Department of First Hospital of Harbin Medical University. RPMI 1640 was purchased from GIBCO. Propidium iodide and Rnase were from Sigma Chemical Co. A murine monoclonal antibody against human Bcl-2 and Bax oncoprotein and antimice rabbit polyclonal antibody were purchased from Maixin Co., Fuzhou.
Cell culturing Human hepatoma BEL-7402 cells were grown as monolayers in RPMI 1640 medium supplemented with 8% calf serum (CS), 100 IU/mL penicillin and 100 mg/mL streptomycin and incubated at 37 °C in the humidified incubator with 5% CO2/95% air. The exponent growing BEL-7402 cells were suspended in medium, planted 4-6 × 105/flask or 3 × 104/well into culture flask or 24-well plate.
Drug treatment The growing BEL-7402 cells planted into culture flasks or 24-well plates were incubated at 37 °C in 5% CO2/95% air for 24 h. The medium was aspirated and replaced with medium containing arsenic trioxide (final concentration 0.5 µmol/L, 1 µmol/L, 2 µmol/L, respectively) as treatment groups and medium with non-arsenic trioxide as controls.
Determination of growth curve The human hepatoma BEL-7402 cells were digested with 0.25% trypsin, stained with 2% trypan-blue. The cell growth and proliferation of BEL-7402 cells were observed by counting the cell number every day. The non-blue-stained cells were viable cells or apoptotic cells and the blue-stained cells were necrotic cells.
Morphologic observation The BEL-7402 cells growth and morphologic changes were observed by OLYMPUS IX70 inverted phase-contrast microscopy. After incubated in medium containing 0.5 µmol/L, 1 µmol/L, 2 µmol/L arsenic trioxide (experimental groups) and non-arsenic trioxide (control groups) for 4 successive days, the cells were prefixed in 2.5% glutaraldehyde, postfixed in 1% OsO4, dehydrated in ethanol series, and replaced in propene oxide. The cell samples attached to grids were examined with a JEM-1220 transmission electron microscope (TEM) and the cell samples grown on covered glass-slide (made in non-enzyme digestion) were gilded in vacuum and examined with a HITACHI S-520 scanning electron microscope (SEM).
Flow-cytometry analysis[1] After incubated in medium containing varying concentrations of arsenic trioxide and in the control groups for 4 d, the cells were digested with 0.25% trypsin, collected after centrifugation at 1000 rpm for 10 min, than washed two times with cold PBS by centrifugation (1000 rpm, 5 min), resuspended in 0.5 mL PBS, adjusted to a cell concentration of 1 × 106/L, and fixed with ice-cold 70% alcohol at 4 °C (could the preserved for less than 2 weeks). The fixed water of single-cell suspension was discarded after centrifuged at 1000 rpm for 10min, which was them washed twice with PBS (1000 rpm, 5 min), and adjusted to a cell concent ration of 1 × 106/L, The DNA was stained with propidium iodide at 4 °C for 30 minutes, then the suspension was analyzed on the apparatus at room temparature. According to ModFit LT software, the cells were divided into four parts: subG1 phase, G0/G1 phase, S phase and G2/M phase.
Immunocytochemistry The BEL-7402 cells of experimental groups and controls were seeded into 24-well plate (with cover glass-slide). After incubated in medium with 0.5 µmol/L, 1 µmol/L, 2 µmol/L arsenic trioxide respectively and non-arsenic trioxide for 4 d, the BEL-7402 cells were fixed with pure acetone for 10 min, washed three times with PBS, acted upon by 0.25% 0.5% Triton X-100 for 10 min, and washed Thrice with PBS. Bcl-2 and Bax protein in the BEL-7402 cells were detected with SABC method. The cell specimens were incubated with 0.3% hydrogen peroxide in methanol for 30 minutes to block the endogenous peroxidase activity, then washed in PBS and incubated in 10% normal goat serum for 20 minutes to reduce nonspecific antibody- binding. Specimens were then incubated with a 1:50 dilution of murine monocional antibody against human Bcl-2 or Bax oncoprotein overnight at 4 °C, followed by washes Thrice with PBS, then incubated with biotinylated rabbit antimice polyclonal antibody at a dilution of 1:100 for 30 minutes followed by another 3 washes. Slides were then treated with streptoavidin-peroxidase reagent for 30 minutes at a dilution of 1:100 and were washed with PBS 3 times. Finally, slides were incubated in phosphate-buffered saline containing diaminobenzidine and 1% hydrogen peroxide for 10 minutes, counterstained with hematine, and mounted.
Statistics All the data were expressed as mean ± standard deviation (x-± s), the differences between the rates of different groups were analysed by χ2 test.
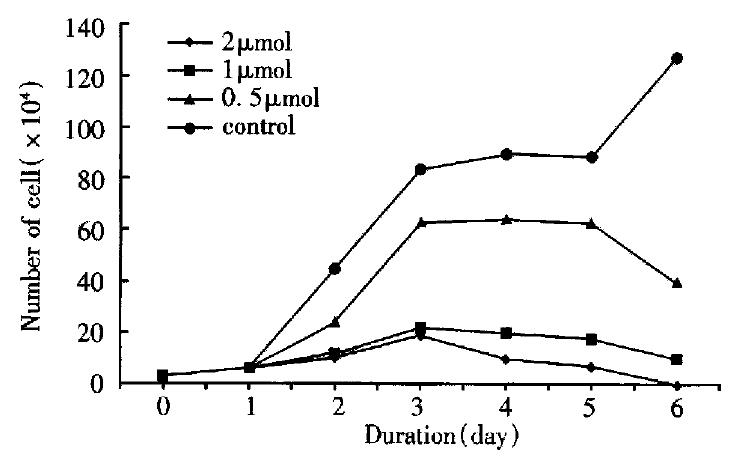
The BEL-7402 cells were incubated in medium containing 0.5 μmol/L, 1 μmol/L and 2 μmol/L arsenic trioxide respectively for 1 to 6 d. The cell-growth inhibitory effect of arsenic trioxide on cells was significant as revealed by cell counting, which was both dose-and time-dependent. During incubated in arsenic trioxide for 3 to 5 d, the speed of growth of BEL-7402 cells was remarkedly slow, on the 6th day, the number of cells fell to the lowest level. The percentage of necrotic cells was less than 1% by trypan-blue staining (Figure 1).
By inverted phase-contrast microscopy, one could find the attaching ability of BEL-7402 cells to the flask treated with 1 μmol/L, 2 μmol/L arsenic trioxide was weaker as compared with that of the controls, and the growth markedly inhibited.
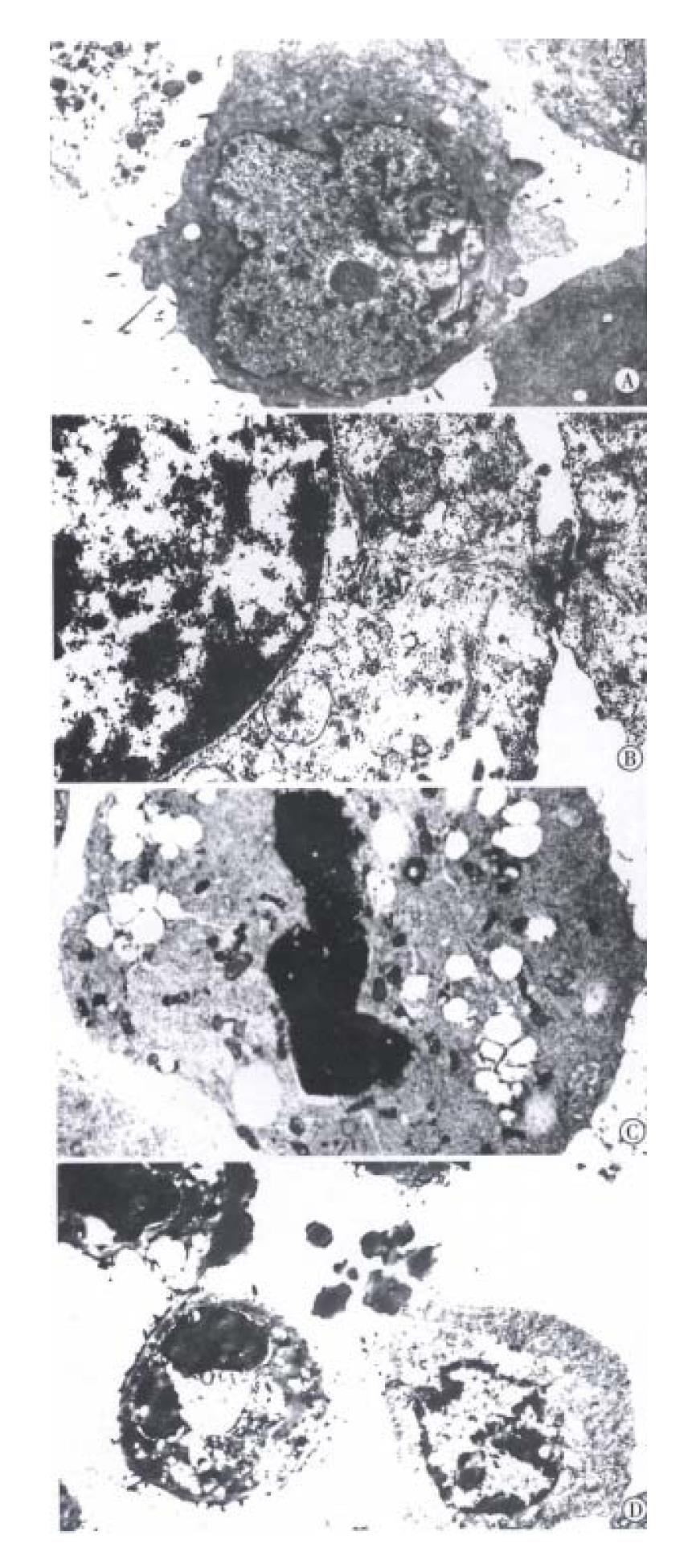
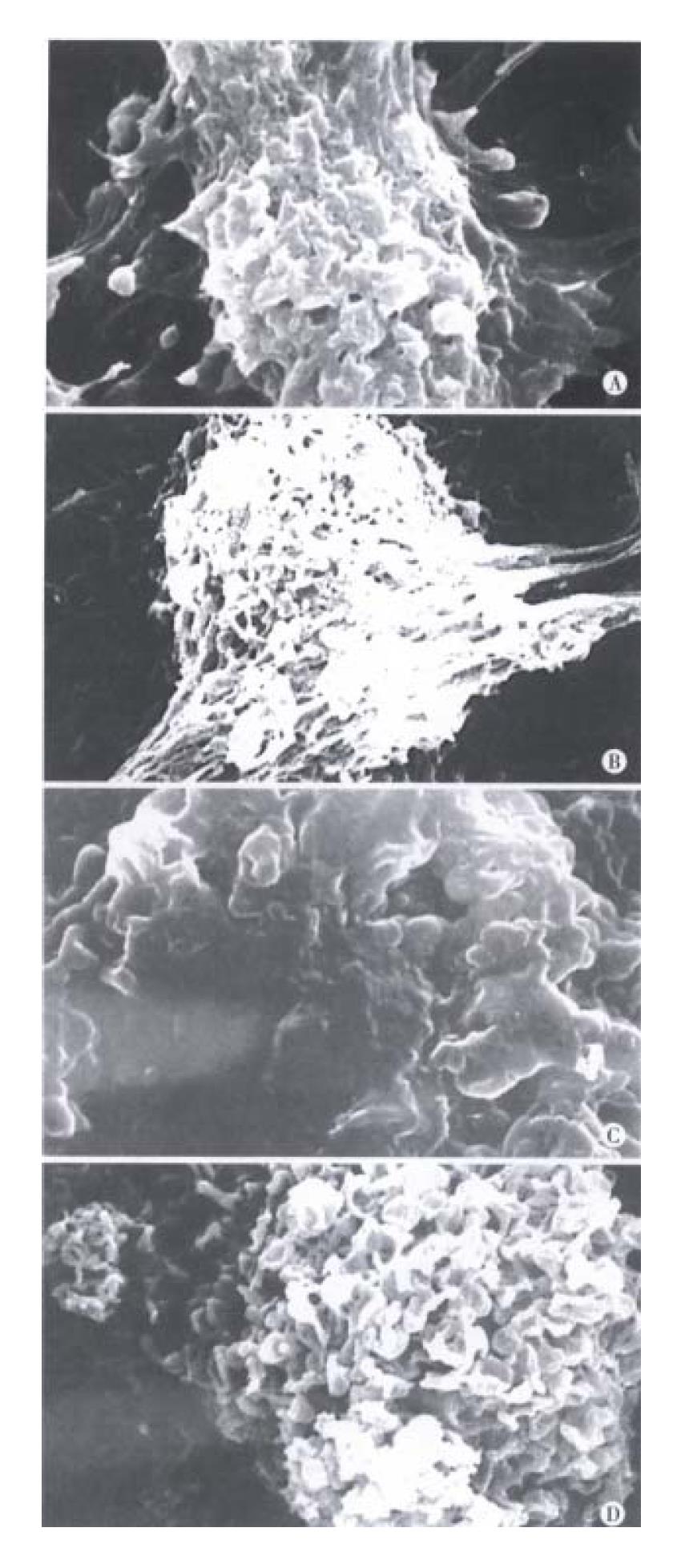
The BEL-7402 cells treated with arsenic trioxide underwent significant changes as seen in TEM, the nucleocytoplasmic ratio in BEL-7402 cells enlanged, with indentation of nuclei. The nucleocytoplasmic ratio in BEL-7402 cells treated with 0.5 μmol/L arsenic trioxide was much smaller than that in controls, and the nuclei appeared round, with loss of nuclear indentation, but with well-differentiated organelles in the cytoplasm. When treated with arsenic trioxide at 1 μmol/L, 2 μmol/L for 4 successive days, one could find intact cell membrane, nuclear condensation and apoptotic body formation, also, marked changes on the cell surface in SEM, there were abundant processes and microvilli on the surface of BEL-7402 cells in the control group, but much less in 0. 5 μmol/L arsenic trioxide group. The processes and microvilli were lost when treated with 1 μmol/L and 2 μmol/L arsenic trioxide (Figure 2, Figure 3).
After treated with 0.5 μmol/L, 1 μmol/L, 2 μmol/L arsenic trioxide for 4 successive days, the BEL-7402 cells were analysed by flow-cytometry. The apoptotic rate of cells in the control group was 9.31%, in 0.5 μmol/L arsenic trioxide group, 15.53%, there was no statistical difference between the two (P > 0.05). The apoptotic rates of BEL-7402 cell in 1 μmol/L and 2 μmol/L arsenic trioxide were significantly higher than that of the control group, being 19.10% and 21.89%, respectively (P1 < 0.05, P2 < 0.05) (Table 1).
After treated with arsenic trioxide at 0.5 μmol/L, 1 μmol/L and 2 μmol/L for 4 successive days, the DNA distribution of BEL-7402 cells showed great changes. The percentage of G0/G1 phase cell in the control group was 62.91%, that of S phase cell, 33.77%. The percentage of G0/G1 phase cell of the experimental groups decreased progressively more than that of the control groups with increase of concentration of arsenic trioxide, whereas the percentage of S phase cell was increased, but with no statistical difference between the two (P > 0.05) (Table 1).
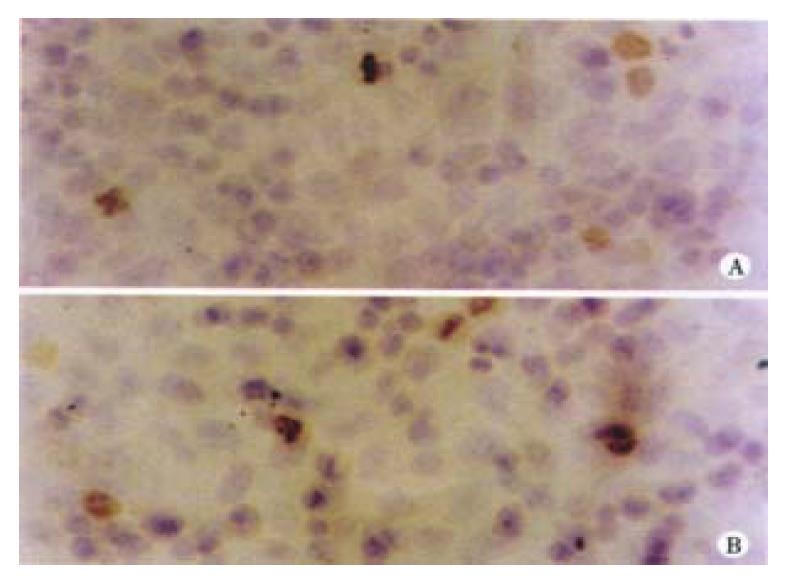
After treated with 0.5 μmol/L, 1 μmol/L, 2 μmol/L arsenic trioxide for 4 successive days, the expression of Bcl-2 and Bax protein were detected by immunocytochem istry. The positive expression rates of Bcl-2 in 1 μmol/L and 2 μmol/L arsenic trioxide were 46.00% and 83.08%, respectively, significantly higher than that in the control group (P < 0.01). This was also higher in 0.5 μmol/L arsenic trioxide group, yet there was no statistical difference between them (P > 0.05). The positive expression rate of Bax in 0.5 μmol/L arsenic trioxide was significantly lower than that of the control group (P < 0.01), but it was significantly higher in 1 μmol/L, 2 μmol/L groups than that in the control group (P1 < 0.01, P2 < 0.01) (Table 2 and Figure 4).
It has been demonstrated that in vitro study is superior to in vivo, study, being also in consistency with the latter therefore, it is the main method in studying the effect of anti-tumor drugs currently[8].
Inhibition of proliferation is the basic reguirement of anticancer drugs[9-16]. Recent clinical studies in China showed that arsenic trioxide is an effective and relatively safe drug in the treatment of acute promyelocytic leukemia. Chen[17] found arsenic trioxide could trigger apoptosis of APL cell line NB4 cells, which was associated with downregulation of bcl-2 gene expressions and modulation of PML-RAR alpha chimeric protein. Advanced hepatocel lular carcinoma has limited, treatment options and prognosis is poor. Recent studies have focused on the apoptosis either by suppression of Bcl-2, Bcl-xL or promotion of Bik, Bax, Bak[18-24]. Our results showed the BEL-7402 cell growth was significantly inhibited by arsenic trioxide at the concentrations of 0.5 μmol/L, 1 μmol/L and 2 μmol/L, and the inhibition effect was not relevant to the cytotoxicity as shown by cell counting after trypan-blue staining. When incubated with arsenic trioxide for 3 to 5 days, the speed of growth of the BEL-7402 cells was remarkably slow. Funthermore, the percentage of G0/G1 phase cells was lower than that of the control group, the apoptotic rate of the experimental group was significantly higher, These indicated the inhibition effect of arsenic trioxide acted mainly on the G0/G1 phase cells. The percentage of S phase cells was much increased, suggesting there might be synergistic effect when arsenic trioxide used in combination with other S-phase anti-cancer drugs.
Apoptosis in a complicated physio-pathologic process involving the suicidal mechanism[25]. Many anticancer drugs can induce apoptosis of neoplastic cells, which opens a new avence to treatment of cancer[26]. Modern mole cular biological investigations have indicated that apoptosis is regulated by many oncogenes, such as p53, c-myc, bcl-2, bax, bad etc.[27-32]. Bcl-2 in an apoptosis-related gene and plays an important role in regulating apoptosis[33-38], by blocking the final pathwayof apoptotic signal transmitted system[39-44]. Bcl-2 can inhibit the apoptosis stimulated by chemotherapeutic drugs, radiotherapy, heat shock, free radicals, Ca2+ and TNF etc.[45-50]. Bax is a homologous protein of Bcl-2, in the form of homopolymer or isodipolymer Bcl-2/Bax[51-54]. The ratio of Bax and Bcl-2 protein influences the apoptotic rate of cells stimulated by several external or internal factor[55,56]. Chen’s report showed that arsenic trioxide did not influence bax, bcl-x, c-myc, or p53 gene expression, but downregulates bcl-2 gene expression at both mRNA and protein levels[56].
Our results suggested that the proliferation inhibitory effect of arsenic trioxide at 1 μmol/L and 2 μmol/L was related to apoptosis induction which was possibly regulated by the expression of Bcl-2, Bax. Oltval et al[29] reported the number of apoptotic cells was higher when Bax protein was predominant. In our study, the apoptotic percentage by flow-cytometry assay was concordant with the protein expression level of Bcl-2 and Bax in 1 μmol/L and 2 μmol/L arsenic trioxide-treated cells.
Arsenic trioxide has dual effects on APL cells: preferential apoptosis at high concentration (0.5-2 μM) and partial differentiation at low concentration (0.1-0.5 μM)[57]. Treatment of BEL-7402 cells with 0.5 μmol/L arsenic trioxide showed intricate results in our study. The reasons were as follows: ① The apoptotic rate of BEL-7402 cell increased after 0.5 μmol/L arsenic trioxide treatment, but had no statistical difference as compared with that of the control. Expressi on of the Bcl-2 protein was increased with arsenic trioxide treatment, whereas expression of Bax protein fell. Ogasawara et al[58] confirmed that Fas system played an important role in hepatocytic apoptosis induction, therefore, other apoptosis-related gene expression might be involved in that treated with 0.5 μmol/L arsenic trioxide. ② It had been seen that the nucleocytoplasmic ratio was decreased, the nucleus became round, with loss of indentation but there were well-differentiated organelles in the cytoplasm, suggesting a possible role for 0.5 μmol/L arsenic trioxide in the induction of differentiation.
In conclusion, our study demonstrates the proliferation inhibition and apoptosis induction effects of arsenic trioxide at the concentrations of 0.5 μmol/L, 1 μmol/L and 2 μmol/L on the human hepatoma cell line BEL-7402. It seems that arsenic trioxide can be a new adjuvant drug in treatment of liver cancer. Arsenic trioxide can be introduced into human body via hepatic artery catheterization to induce cell apoptosis. The present study provides some theoretical basis for its clinical use.
Edited by Wu XN Proofread by Ma JY
| 1. | Zhang P, Wang SY, Hu LH. 72 Acute promyelocytic leukemia treated with arsenic trioxide. Zhonghua Xueyexue Zazhi. 1996;17:58-60. |
| 2. | Andre C, Guillemin MC, Zhu J, Koken MH, Quignon F, Herve L, Chelbi-Alix MK, Dhumeaux D, Wang ZY, Degos L. The PML and PML/RARalpha domains: from autoimmunity to molecular oncology and from retinoic acid to arsenic. Exp Cell Res. 1996;229:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Zuo LF. Techniques of the preparation of the flow-cytometry sample. Ed 1. Beijing: Hua Xia Press 1989; 48-49. |
| 4. | Omura M, Hirata M, Tanaka A, Zhao M, Makita Y, Inoue N, Gotoh K, Ishinishi N. Testicular toxicity evaluation of arsenic-containing binary compound semiconductors, gallium arsenide and indium arsenide, in hamsters. Toxicol Lett. 1996;89:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wang ZY. Development of arsenical clinical application and study of arsenical mechanism. Zhonghua Xueyexue Zazhi. 1996;17:57. |
| 6. | Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ. Use of arsenic trioxide in the treatment of acute promyelocytic leuke mia (APL): I As-2O-3 exerts dose dependent dual effects on APL cells. Blood. 1997;89:3354-3360. |
| 7. | König A, Wrazel L, Warrell RP, Rivi R, Pandolfi PP, Jakubowski A, Gabrilove JL. Comparative activity of melarsoprol and arsenic trioxide in chronic B-cell leukemia lines. Blood. 1997;90:562-570. [PubMed] |
| 8. | Chen WC, Pan SC. Application of human cancer cells cultured in vitro on cancer study. Guowai Yixue Fenzi Shengwuxue Fenche. 1992;12:1035. |
| 9. | Tan LJ, Chen XY, Shen ZY, Zhang L, Cai WJ. Study on the prolifera-tive inhibition of Human Esophageal cancer cells with treatment of DMSO and As2O3. Shanghai Dier Yike Daxue Xuebao. 1999;19:5-8. |
| 10. | Guo WJ, Yu EX, Zheng SG, Shen ZZ, Luo JM, Wu GH, Xia SA. Study on the apoptosis and cell cycle arrest in human liver cancer SMMC7721 cells in duced by Jianpiliqi herbs. Shijie Huaren Xiaohua Zazhi. 2000;8:52-55. |
| 11. | Chen HY, Liu WH, Qin SK. Induction of arsenic trioxide on apoptosis of hepatocarcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:532-535. |
| 12. | Tu SP, Jiang SH, Tan JH, Jiang XH, Qiao MM, Zhang YP, Wu YL, Wu YX. Proliferation inhibition and apoptosis induction by arsenic trioxide on gastric cancer cell SGC 7901. Shijie Huaren Xiaohua Zazhi. 1999;7:18-21. |
| 13. | Shen YF, Zhuang H, Shen JW, Chen SB. Cell apoptosis and neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:267-268. |
| 14. | Liang YR, Wang CF, Zhou JH, Peng XZ. Apoptosis of hepatocyte and precancerous lesion of hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:160-162. |
| 15. | He SW, Shen KQ, He YJ, Xie B, Zhao YM. Regulatory effect and mechanism of gastrin and its antagonists on colorectal carcinoma. World J Gastroenterol. 1999;5:408-416. [PubMed] |
| 16. | Li J, Wang WL, Wang WY, Liu B, Wang BY. Apoptosis in human hepatocellu lar carcinoma by terminal deoxynucleotidyl transferase mediate dUTP FITC nick end labeling. Huaren Xiaohua Zazhi. 1998;6:491-494. |
| 17. | Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345-3353. [PubMed] |
| 18. | Wang XM, Wang X, Li J, Evers BM. Effects of 5-azacytidine and butyrate on differentiation and apoptosis of hepatic cancer cell lines. Ann Surg. 1998;227:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223-227. [PubMed] |
| 20. | Liang YR, Zheng SY, Shen YQ, Wu XY, Huang ZZ. Relationship between expression of apoptosis related antigens in hepatocellular carcinoma and in situ end labeling. World J Gastroentero. 1998;4:99. |
| 21. | Zhuang XQ, Yuan SZ, Wang XH, Lai RQ, Luo ZQ. Oncoprotein expression and inhibition of apoptosis during colorectal tumorigenesis. China Natl J New Gastroenterol. 1996;2:3-5. |
| 22. | Huang PL, Zhu SN, Lu SL, Dai ZS, Jin YL. Inhibitor of fatty acid synthase induced apoptosis in human colonic cancer cells. World J Gastroenterol. 2000;6:295-297. [PubMed] |
| 23. | Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6:263-265. [PubMed] |
| 24. | Wang LD, Zhou Q, Wei JP, Yang WC, Zhao X, Wang LX, Zou JX, Gao SS, Li YX, Yang C. Apoptosis and its relationship with cell proliferation, p53, Waf1p21, bcl-2 and c-myc in esophageal carcinogenesis studied with a high-risk population in northern China. World J Gastroenterol. 1998;4:287-293. [PubMed] |
| 25. | Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359-361. |
| 26. | Su J. Progression apoptosis of neoplasm cell induce by drugs. Guowai Yixue Zhongliuxue Fenche. 1995;22:7-10. |
| 27. | Kong XP, Zou QY, Li RB, Zheng PL, Yang LP, Jin SW. Apoptosis of neoplasm cell lines induced by hepatic peptides extracted from sucking porcine hepatocytes. World J Gastroenterol. 1999;5:435-439. [PubMed] |
| 28. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4503] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 29. | Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1574] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 30. | Dai J, Yu SX, Qi XL, Bo AH, Xu YL, Guo ZY. Expression of bcl 2 and c-myc protein in gastric carcinoma and precancerous lesions. World J Gastroentero. 1998;4:84-85. |
| 31. | Cao GD, Wang SW, Wu SS, Li HF, Zhang WG. Retrovirus mediated antisense RNA to bcl-2 alter the biological behavior of stomach carcinoma MGC 803 cell lines. World J Gastroentero. 1998;4:45-48. |
| 32. | Wang XW, Xie H. Presence of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells. World J Gastroenterol. 1998;4:540-543. [PubMed] |
| 33. | Chen RF, Zou SQ, Qian JQ. Apoptosis and expression of bcl-2 gene in gallbladder neoplasms. Huaren Xiaohua Zazhi. 1998;6:680-682. |
| 34. | Peng XM, Peng WW, Chen Q, Yao JL. Apoptosis, Bcl 2 and P53 protein xpressions in tissues from hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:834-836. |
| 35. | Wang JM, Zou Q, Zou SQ. The role of bcl-2 gene in apoptosis of liver in rat with obstructive jaundice. Shijie Huaren Xiaohua Zazhi. 1999;7:1035-1037. |
| 36. | Qiao Q, Wu JS, Zhang J, Ma QJ, Lai DN. Expression and significance of apoptosis related gene bcl-2, bax in human large intestine adenocarcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:936-938. |
| 37. | Li XL, Hao YR, Zou JX, Yang JH, Geng JH. Relationship between C-myc and Bcl-2 alterations and biological behavior and apoptosis in gastric cancer. Xin Xiaohuabingxue Zazhi. 1997;5:773-774. |
| 38. | Guo LL, Cao CA, Wang YS. The study of the expression of Bcl-2 and Bax in hepatocellular carcinoma. Xin Xiaohuabingxue Zazhi. 1997;5:655-656. |
| 39. | Raffo AJ, Perlman H, Chen MW, Day ML, Streitman JS, Buttyan R. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995;55:4438-4445. [PubMed] |
| 40. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2713] [Article Influence: 77.5] [Reference Citation Analysis (1)] |
| 41. | Mandal M, Wu X, Kumar R. Bcl-2 deregulation leads to inhibition of sodium butyrate-induced apoptosis in human colorectal carcinoma cells. Carcinogenesis. 1997;18:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Boyd JM, Gallo GJ, Elangovan B. Bik, a novel death inducing protein,shares a distinct sequence motif with bcl-2 family proteins and interacts with viral and cellular survival promoting proteins. Oncogene. 1995;11:1921-1928. |
| 43. | Bellamy CO, Malcomson RD, Harrison DJ, Wyllie AH. Cell death in health and disease: the biology and regulation of apoptosis. Semin Cancer Biol. 1995;6:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Hawkins CJ, Vaux DL. Analysis of the role of bcl-2 in apoptosis. Immunol Rev. 1994;142:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Korsmeyer SJ. Bcl-2: an antidote to programmed cell death. Cancer Surv. 1992;15:105-118. [PubMed] |
| 46. | Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1619] [Cited by in RCA: 1686] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 47. | Nuñez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602-3610. [PubMed] |
| 48. | Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 881] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 49. | Tan XH, Xu AG, Piao YJ, Yang DH. Basis and clinic of cell apoptosis. Ed1. Beijing: Renmin Junyi Press 1999; 19-20. |
| 50. | Walton MI, Whysong D, O'Connor PM, Hockenbery D, Korsmeyer SJ, Kohn KW. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993;53:1853-1861. [PubMed] |
| 51. | Liu HF, Liu WW, Fang DC, Men RP. Expression of bcl-2 protein in gastric carcinoma and its significance. World J Gastroenterol. 1998;4:228-230. [PubMed] |
| 52. | Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15-17. [PubMed] |
| 53. | Liang YR, Zheng SY, Shen YQ, Wu XY, Huang ZZ. Relationship between expression of apoptosis related antigens in hepatocellular carcinoma and in situ end labeling. Huaren Xiaohua Zazhi. 1998;6:236-239. |
| 54. | Li J, Wang WL, Liu B. Angiogenesis and apoptosis in human hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:1057-1060. |
| 55. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax gene expression and its relationship with apoptosis in human gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. |
| 56. | Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052-1061. [PubMed] |
| 57. | Chen Z, Wang ZY, Chen SJ. Acute promyelocytic leukemia: cellular and molecular basis of differentiation and apoptosis. Pharmacol Ther. 1997;76:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |