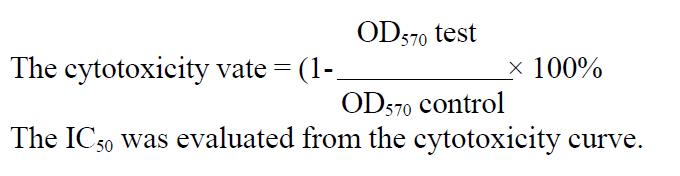INTRODUCTION
Gastric cancer is one of the most common malignant tumors in China. Evidences have demonstrated that stomach cancer is a disease caused not only by excessive cellular proliferation and poor differentiation, but also by decrease in apoptosis of the gastric cells[1]. Though the disease in its early stage can be treated by surgical resection, in advanced stage its response to conventional chemotherapy or radiotherapy is usually not satisfactory. Therefore, we think, that induction of apoptosis of gastric cancer cells might be a new means in the treatment of gastric cancer. Arsenic trioxide (As2O3) and hydroxycamptothecin (HCPT) have long been used in China. The former was first reported by investigators in Harbin and Shanghai to be an effective drug in the treatment of patients with a cute promyelocytic leukemia (APL)[2,3]. It induces apoptosis of the leuk emic cells at a concentration achieved in the plasma of treated patients (0.5 × 10-6 mol/L-1 × 10-6 mol/L), as demonstrated by studies on all-trans-retinoic acid (ATRA)-susceptible or resistant APL cell lines, on primary APL cell culture, and on patients’ blood samples obtained during treatment with arsenic[4]. It is not known whether As2O3 is effective in the treatment of solid tumors such as gastric cancer or not.
HCPT is a unique antitumor drug that has been extracted and synthesized by Chine se scientists from Camptotheca accuminata which is a native plant in China[5]. HCPT can act directly on topoisomerase I inhibiting its activity. HCP T possesses stronger cytotoxicity to tumor cells with less side effects, as com pared to camptothecin[6]. More recent studies have shown that camptothec in have strong apoptosis induction effects in human leukemia cell lines[7]. However, there is yet no report about HCPT-induced apoptosis in stomach cells. Therefore, we studied the effects of HCPT and As2O3 on induction of apoptosis in gastric cancer cells.
MATERIALS AND METHODS
Cell culture and chemicals
Human moderately differentiated gastric adenocarcinoma cell line SGC-7901 was obtained from Shanghai Sixth People’s Hospital, human poorly differentiated sto mach adenocarcinoma cells line MKN-45 and well differentiated stomach adenocarc inoma cell line MKN-28 were kindly provided by Japanese Cancer Research Resources Bank Corp (Tokyo, Japan). SGC-7901, MKN-45 and MKN-28 were maintained in a humidified, 5% CO2 atmosphere and cultured in RPMI 1640 (GIBCO) supplemented with 10% FCS, 2 μmol/L L-glutamine, 100 units/mL penicillin, and streptomycin. HCPT 1 g/L and 0.1% As2O3 preparation for iv administr ation were kindly provided by Hubei Huangshi Second Pharmaceutical Company,and Shanghai Institute of Hematology, respectively.
MTT method and cytotoxicity
One hundred μL cancer cells in exponential growth at 1 × 104/mL were added into flat-bottomed 96-well plates (NUNC) 24 h prior to drug treatment. Cells w ere treated with 1 mg/L-100 mg/L HCPT, (0.5-10) μmol/L As2O3 and with no drugs (control) in triplicate for 24 h and 48 h, respectively. After washing th e medium was replaced by 100 μL RPMI 1640 (GIBCO) medium containing 1 g/L3(4,5dimethylthiazol)-2,5diphenyltetra-zolium(MTT, MERCH). After 4 h , plates were centrifuged at 800 × g for 5 min, the MTT medium was removed, and the blue dye was dissolved in 200 μL of warm dimethylsulfoxide (DMSO). Absorban ce was measured at 570 nm. The cytotoxicity rates were measured by the formula:
Math 1
Apoptosis cell morphology
One hundred μL cancer cells at concentration of 5 × 106/mL were in cubated into 6-well plates with previously placed glass slides. After 24 h, the medium was replaced with the drug containing medium. After incubation with drugs for 12 h, 24 h, 48 h, and 72 h, glass slides with cancer cell growth were fixed with 4% polyformalin, and stained with hematoxylin-eosin. Cell morphology was examined under light microscopy.
Transmission electron microscopy
Culture cells were fixed in 2% glutaraldehyde in 0.1 mol/L, pH 7.4 PBS at 4 °C and postfixed with 1% osmium tetroxide for 2 h, then the cells were embedded with Eppon 812 and ultrathin sections were cut. Cells were observed under transmission electron microscope (H-500, Japan).
TUNEL assay
Apoptosis was assessed by dUTP labeling of DNA nicks with terminal deoxynucleoti dyl transferase (TUNEL). One hundred μL cancer cells at concentr ation of 5 × 106 /mL were inoculated into 6-well plates with previously placed glass slides. After 24 h, the medium was replaced with the drug containing medium. After incubation w i th drugs for 24 h and 48 h, glass slides with cancer cells growth were fixed with 4% polyformalin. The TUNEL assay was performed according to the instructions in the In Situ Cell Death Detection Kit (Boehringer-Mannheim, Germany). Briefly, after washing twice with pH 7.4 PBS, 50 μL TUNEL reaction solution was added to the well, then incubated at 37 °C for 2 h. After substrate reaction, stained cells were examined under light microscopy. Apoptotic cells were scored and expressed as the number of positively stained cells per 500 cells (n = 4-5) .
Flow cytometry
Apoptotic cells were also detected by flow cytometry which was performed as described previously. About 1 × 106 cells were treated with drugs for 24 h and 48 h. After trypsin digestion, the cells were collected by centrifugation, then fix ed in 70% ethanol /phosphate buffered saline for at least 12 h at 4 °C. After 100 μL (1 g/L) RNase treatment, cells were stained with 50 mg/L propidium iodide. Cells were examined by flow cytometry using a FACScan (Becton-Dickinson , USA). The results were analyzed with Lysis II software (Becton-Dickinson).
RESULTS
As2O3 and HCPT cytotoxicity
HCPT and As2O3 had strong cytotoxic effect on gastric cancer cells (Figure 1 ). The IC50 of As2O3 on MKN-28, SGC-7901, and MKN-45 was 8.91 μmol/ L, 10.57 μmol/L, and 11.65 μmol/L, respectively.The IC50 of HCPT on MKN-28, SGC-7901, MKN-45 was 9.35 mg/L, 10.21 mg/L, and 12.63 mg/L, respectively.
Figure 1 Cytotoxicity of As2O3 (A), and HCPT (B), on gastric cancer cells.
Induction of apopotosis by As2O3 and HCPT
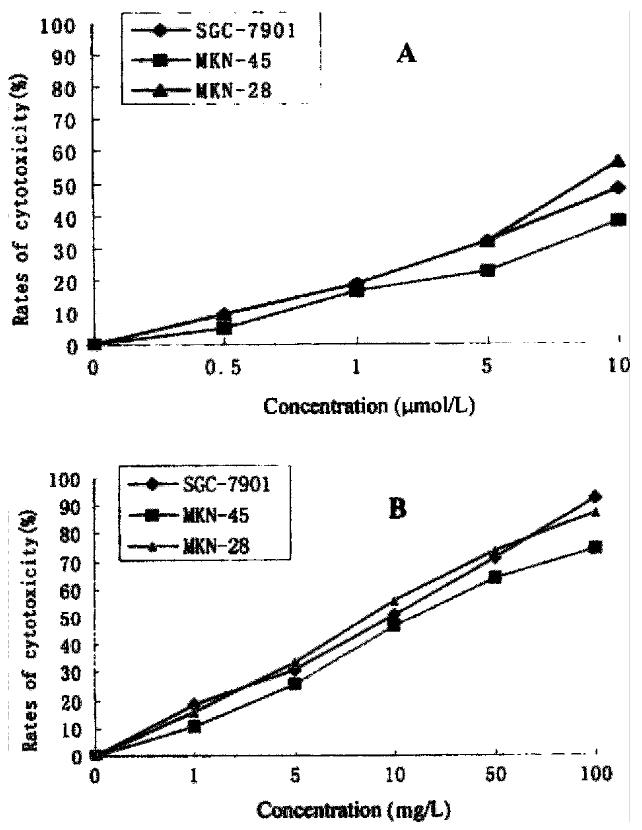
After 12 h of exposure to As2O3 and HCPT, gastric cancer cells MKN-28 and SGC-7901, began to show morphologic features of apoptosis. The apoptotic cells increased on prolongation of exposure time to drugs. The ultrastructural feat ures of apoptosis was observed in MKN-45 and SGC-7901 by transmission electron microscopy, including cell shrinkage, cytoplasmic blebs, condensation of chromatin, nuclear condensation, fragmentation of nucleus, and formation of apoptotic bodies, and so on. The results are presented in Figure 2.
Figure 2 Electron microscopic observation of ultrastructural changes in gastric cancer cells treated with 10 μmol/L As2O3 for 48 h.
The early change of apoptosis, nuclear chromatin condensation, looks like a new moon subjacent to the nuclear membranes (A). The intermediate stage of apoptosis presents cytoplasmic blebs, nuclear condensation, overflow of nuclear chromatin, and like-sprout (B). The late stage of apoptosis is characterized by splitting of nuclear membrane, nuclear chr omatin condensation, overflow of some parts of nuclear chromatin, and fragmentation of nucleus (C), and formation of apoptotic bodies (C) (D).
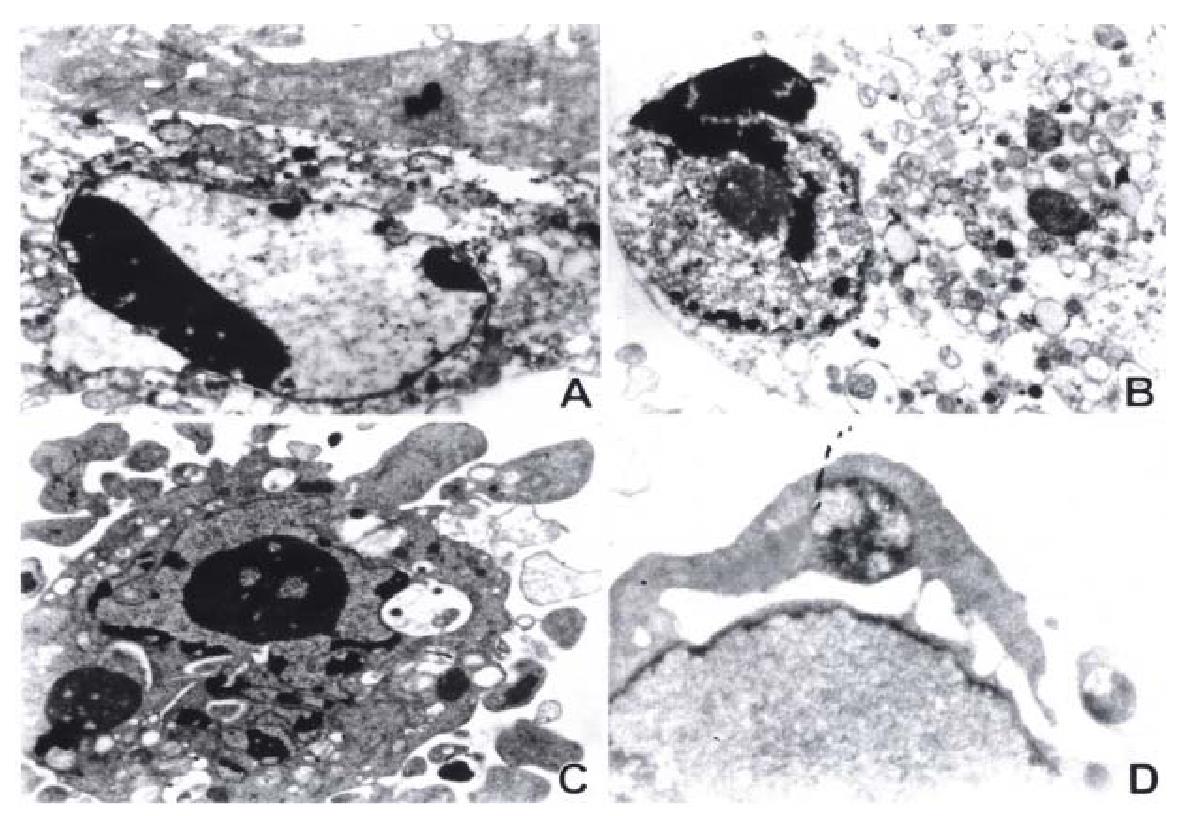
As2O3 and HCPT induced time- and dose-dependent apoptosis in three strains of gastric cancer cells. The indice of apoptosis were measured by TUNEL. The results are presented in Figure 3A and 3B.
Figure 3 The index of apoptosis of gastric cancer cells induced by As2O3 (A) and by HCPT (B).
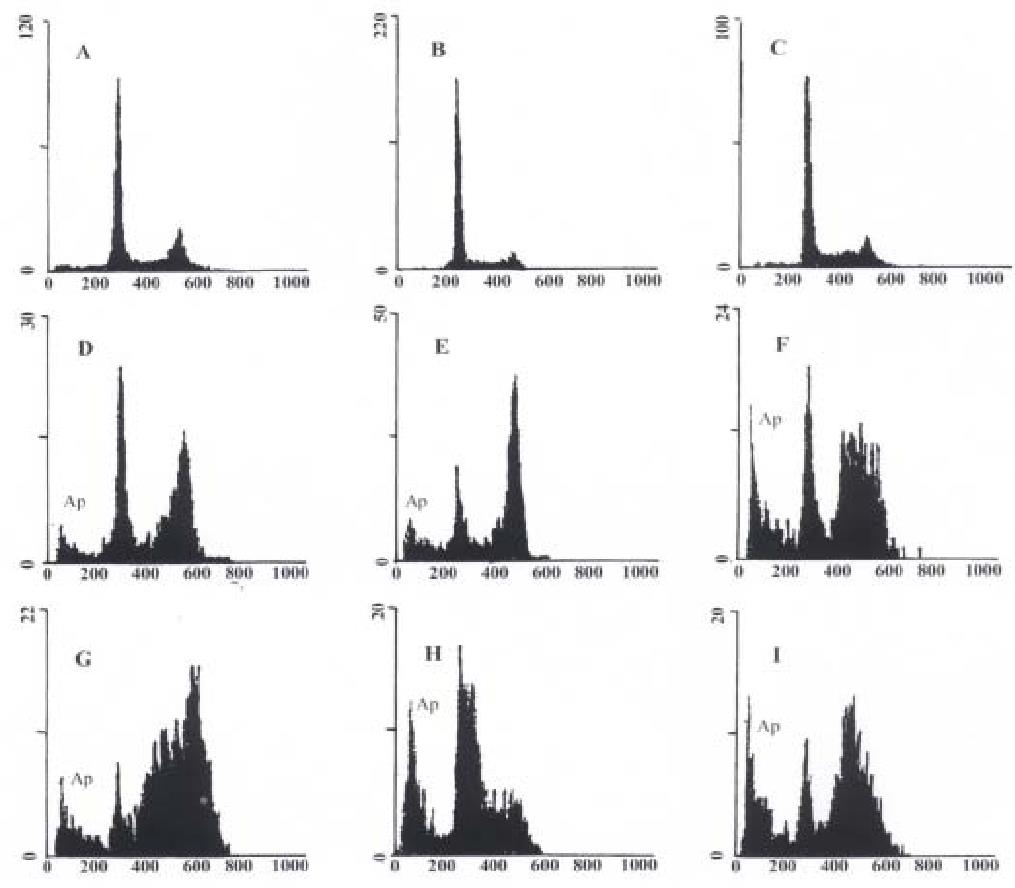
To further study the inducing effect of As2O3 and HCPT on gastric cancer cells, we analysed the DNA fragment reflecting the endonuclease activity during apoptosis. (Figure 4). Examination of histogramic related nuclear DNA contents on FACS showed a distinct region below G1 phase, which is the typical profile of apoptotic cells in which DNA stainability is reduced due to degradation and subsequent leakage of DNA from cells. After 48 h exposure to 10 mg/L HCPT, the apoptotic rates of SGC-7901, MKN-45, and MkN-28 were 21.88%, 12.35%, and 30.26%, respectively. After 48 h of exposure to 10 μmol/L As2O3, the apoptotic rates of SGC-7901, MKN-45, and MKN-28 were 13.84%, 22.52%, and 9.68%, respectively (Figure 4).
Figure 4 Nuclear DNA contents measured by flow cytometry in As2O3 and HCPT-induced apoptosis in gastric cancer cells at 48 h.
Ap represents apoptotic cells. A: Untreated MKN-45 cells; B: Untreated SGC-7901 cells; C: Untreate d MKN-28 cells; D: MKN-45 cells treated with 10 μmol/L As2O3; E: SGC-79 01 cells treated with 10 μmol/L As2O3; F: MKN-28 cells treated with 10 μmol/L As2O3; G: MKN-45 cells treated with 10 mg/L HCPT; H: SGC-7901 cells treated with 10 mg/L HCPT; I: MKN-28 cells treated with 10 mg/L HCPT.
Effect of As2O3 and HCPT on cell cycle of SGC-7901 cells
The effect of As2O3 and HCPT on SGC-7901 cells show remarkable cell cycle specificity. There was no significant change in cell cycle after 10 μmol/L As2O3 treatment for 24 h. The fraction of G0/G1 was decreased from 54.2% to 17.7%, while the fraction of G2/M was significantly increased from 20.2% to 63.4% with 10 ¦Ìmol/L AsO treatment in SGC-7901 cells for 48 h. The results showed that As2O3 induced gastric cancer cell death was in G1 phase, blocked at G2/M phase. The fraction of G0/G1 phase was decreased from 54 .2% to 37.6%, while the fraction of S phase was increased from 25.6% to 38.6% with 10 mg/L HCPT treatment in SGC-7901 cells for 48 h. The results showed that HCPT-induced apoptosis was at G1 phase, and arrested at S phase.
DISCUSSION
Since the first observation of the relationship between arsenic and skin cancer in 1820s, arsenic compounds have been generally recognized as potent environ mental carcinogens, more likely as a co-mutagen and co-carcinogen for human sk in and lungs[8], although no animal model had been established[9]. Biochemically, it is documented that arsenic can inactivate some important enzymes by binding their sulfhydryl groups. Arsenic can also interfere with the phosphorylation-dephosphorylation process by replacing the phosphorylation reaction. It has also been shown that arsenic can induce chromosome aberrations, sister-chromatin exchanges, DNA-protein cross links and protein-associated DNA-strand breaks in mammalian cells[10,11]. However, low concentration of some arsenic compounds also had some benefits to human physiologically,such as stimulation of human hematopoiesis. The use of arsenic compounds as drugs has a long history in Chinese traditional medicine. For example, it was recorded that ar senic had therapeutic effects on some human diseases such as psoriasis, syphilis, and rheumatosis. Recently, it has been shown that two arsenic compounds As2O3[2] and arsenic disulfide[12], which were used in some tradit ional Chinese prescriptions, are very effective in APL treatment. For instance, a report from the northeastern region of China showed that As2O3 (10 g/L via intravenous infusion for 28 to 60 days ) induced clinical complete remission (CR) in 65.6% of APL patients. More interestingly, 28.2% (9/32) of patients survived more than 10 years. A more recent clinical trial with As2O3 trea tment also demonstrated that CR was achieved in 15 of 16 APL patients who relapsed after ATRA-induced and chemotherapy-maintained CR[4].
It has been suggested that As2O3 might induce apoptosis selectively in APL cells[13]. Furthermore, at pharmacological concentrations, it has no effect on the growth and survival of the leukemia myeloid cells U937 and HL60[3]. However, preliminary reports suggest that the apoptotic effect of As2O3 is not specific for APL cells but can be observed in various lines of either myeloid or lymphoid origin[14-18]and in blast cells from patients wi th non-M3 acute myeloid leukemia [19]. Another arsenic-containing compound, the melaminyl-phenyl-arsenoxide melarsoprol, which is used in the treatment of human African trypanosomiasis, has a broad efficacy against leukemia cells of both lymphoid and myeloid lineage[20]. Both As2O3 and melarsoprol also markedly induce apoptosis in plasma cell lines and in plasma cells from multiple myeloma[21]. Recent reports have demonstrated that As2O3 can induce apoptosis in various cell lines of solid tumor. As2O3 inhibited the growth and survival of solid tumor cell lines in gastric[22], esop hageal[23,24], lung[24], cervix[25], liver[26] tumor and neuroblastoma[27] by triggering programmed cell death. These re sults suggest that As2O3 may be an effective drug in treatment of solid tumors. As2O3 has been intensively investigated for the treatment of cancer.
There is growing evidence indicating that apoptosis plays a crucial role in both carcinogenesis and developement of stomach tumors[1,28-30]. Induct ion of apoptosis in gastric cancer cells might be a new means in the treatment of gastric cancer. It was reported that both radiation and chemotherapy (5-fluorouracil, cisplatinum, mitomycin and trichosanthin) can induce apoptosis in gastric cancer[31-33]. Our previous study showed that As2O3 had an effect on inhibiting proliferation and inducing apoptosis in gastric cancer cell SGC-7901[22]. In this study, we found that As2O3 exhibited a dose-and time-dependent cytotoxicity on gastric cancer cells at different degrees of differentiation. The IC50 of As2O3 on MKN-28, SGC-7901, and MKN-45 were 8.9 μmol/L, 10.5 μmol/L, and 11.6 μmol/L, respectively. Toxicity of As 2O3 was not significantly different among gastric cancer cells at different de grees of differentiation. As2O3-treated gastric cancer cells presented char acteristic morphological changes of apoptotic cells. Results showed DNA degrada tion into oligonucleosomal fragments and these changes occurred in a time-and dose-dependent manner. The apoptotic index was 7%-15% as assessed by TUNEL. A typical subdiploid peak before G0/G1 phase was observed by flow cytometry. After 48 h of exposure to As2O3, the apoptotic rates of SGC-7901, MKN-45, and MKN-28 were 13.8%, 22.5%, and 9.68%, respectively. We concluded th at one of the main effects of As2O3 on gastric cancer cells at the concentrations u sed is to induce cell death by apoptosis.
It was recently shown[45] that the cell cycle time was prolonged in As2O3 treated malignant lymphocytes Namalwa and Raji cells, and that no substan tial increase in cell cycle time was found in Jurkat cells treated with As2O3, as compared with untreated cells. This result suggests that As2O3 can inhibit proliferation of some malignant lymphocyte cell lines by prolonging the cell cycle instead of arresting cells in a specific phase. In the present study, we demonstrated that the effect of As2O3 on SGC-7901 showed remarkable cell cycle specificity in inducing cell death at G1 phase, and blocking prolifer ation at G2/M phase. This result is consistent with the report of Deng et al[46], in which As2O3 induced apoptosis in HeLa cells by arresting G2/M phase of the cell cycle.
Camptothecin and its analogues are agents with a unique spectrum of antitumor activity mediated by a selective inhibition of eukaryotic DNA topoisomerase I (Topo I). The cytotoxicity of these compounds is pr edominantly exerted during S phase, and is associated with an inhibition of DNA replication. This inhibition is generally thought to be mediated by stablization of the CPT-Topo I-DNA cleavable complex[34]. Recent studies have shown that camptothecin and its analogues can strongly induce apoptosis in human leukemic cells[35-36], prostate[36-37], colon[38] and breast[39-40] cancer cells as well as glioma cells[41].
HCPT has been used in the treatment of gastrointestinal tumor[42,43], but its mechanism of anticancer action is still not completely understood. Recently, there were reports that HCPT can induce apoptosis of cancer cells of colon[47], pancreas[48], and bladder[49]. Our previous study showed HCPT can induce apoptosis in gastric cancer cell SGC-701[44]. In this study, we found that HCPT had a strong dose-and time-dependent cytotoxicity to gastric cancer cells at different degrees of differentiation. The IC50 of HCPT on MKN-28, SGC-7901, and MKN-45 was 9.35 mg/L, 10.21 mg/L, and 12.62 mg/L, respectively. There was no significant difference in toxicity of HCPT on gastric cancer cells at different degrees of differentiatio n. Gastric cancer cells treated with HCPT presented characteristic morphological changes of apoptosis. The effects of inducing apoptosis in gastric cancer cells could be co rrelated with time and dosage of HCPT treatment. A typical subdiploid peak before G0/G1 phase was observed by flow cytometry. After 48 h of exposure to 10 mg/L HCPT, the apoptotic rates of SGC-7901, MKN-45, and MKN-28 were 13.8%, 22.5%, and 9.68%, respectively. In this study, the fraction of G0/G1 p hase was decreased from 54. 2% to 37.6%, while the fraction of S phase was in creased from 25.6% to 38.6% with 10 mg/L HCPT treatment of SGC-7901 cells for 48 h. The result suggested that HCPT also showed a remarkable cell cycle specificit y, induced cell death and apoptosis at G1 phase, and arrested proliferation at Sphase.
The molecular mechanism of As2O3 on APL cells showed that the inhibition of cell proliferation was due to a direct induction of apoptosis through downreg ulation of bcl-2 expression and modulation of PML/RARα/PML pro tein in vitro studies[3]. Furthermore, the activation of caspases was also involved in As2O3-induced apoptosis in APL cells[50]. Akao et al also reported that As2O3 induced apoptosis through the down-regulation of Bcl-2 protein and activation of caspases in B-cell leukemia cell lines[51]. It was also reported that As2O3 induced apoptosis in neuroblastoma cell lines through the activation of caspase-3[27]. Litvak et al reported that inhibition of gastric cancer by camptothecin involves apoptosis and multiple cellular pathways, and induction of apoptosis of gastric cancer cell was mediated by up-regulation of p53, p21Waf1/Cip1, and p27Kip1 and the down-regulation of Bcl-2 and Bcl-XL[52]. Camptothecin induced apoptosis thr ough activation of caspase in U-937 cells[53]. Our results showed that HCPT and As2O3 exerted significant cytotoxicity on gastric cancer cells and induction of apoptosis in vitro. The molecular mechanism of apoptotic effect of HCPT and As2O3 on gastric cancer cells the remains to be further investi gated, and the effect of apoptosis must be confirmed by in vivo studies. However , the results of the present study suggest that HCPT and As2O3 might be cand idate drugs to be used in the treatment of gastric cancer, thus needing further studies.