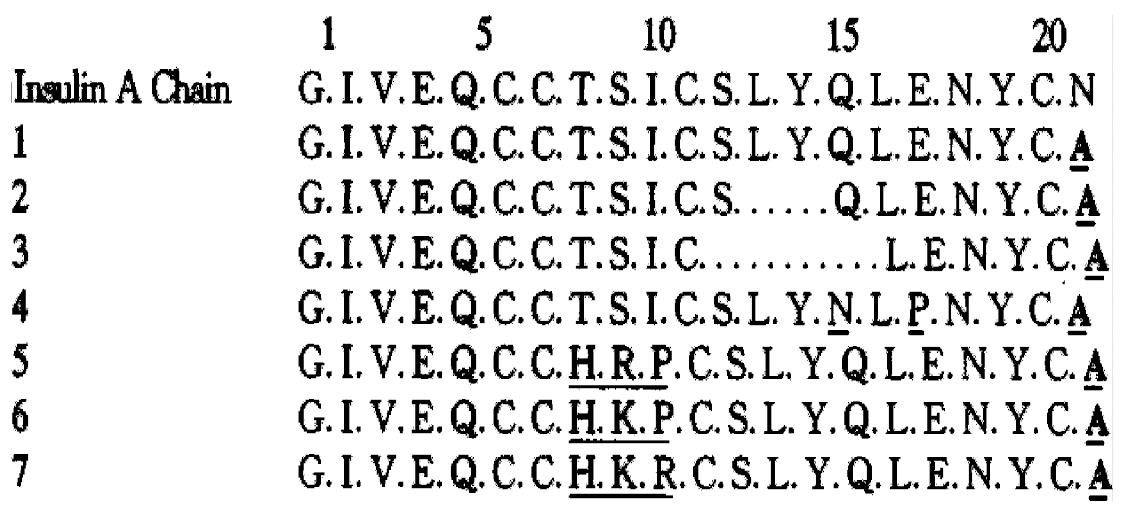Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.371
Revised: February 3, 2000
Accepted: February 29, 2000
Published online: June 15, 2000
AIM: To study the relationship between insulin A chain regions and insulin biological activities, we designed a series of insulin analogues with changes at A21, A12-18 of C-terminal helical region and A8-10 located in the region of A6-A11 intra-chain disulphide bond.
METHODS: Insulin A-chain analogues were prepared by stepwise Fmoc solid-phase manual synthesis and then combined with natural B-chain of porcine insulin to yield corresponding insulin analogues. Their biological activities were tested by receptor binding, mouse convulsion and immunological assay.
RESULTS: [A21Ala]Ins retains 70.3% receptor binding capacity and 60% in vivo biological activity. [DesA13-14, A21Ala]Ins and [DesA12-13-14-15, A21Ala] Ins still have definite biological activity, 7.9% and 4.0% receptor binding, and 6.2% and 3.3% in vivo biological activity respectively. [A15Asn, A17Pro, A21Ala]Ins maintains 10.4% receptor binding and 10% in vivo biological activity. [A8His, A9Arg, A10Pro, A21Ala]Ins, [A8His, A9Lys, A10Pro, A21Ala]Ins and [A8His, A9Lys, A10Arg, A21Ala]Ins have 51.9%, 44.3% and 32.1% receptor binding respectively, 50%, 40% and 30% in vivo biological activity respectively, and 28.8%, 29.6% and 15.4% immunological activity respectively.
CONCLUSION: A21Asn can be replaced by simple amino acid residues. The A chains with gradually damaged structur al integrity in A12-18 helical region and the demolition of the A12-18 helical region by the substitution of Pro and Asn for A17Glu and A15Gln respectively ca n combine with the B chain and the combination products show definite biological activity, the helical structure of A12-18 is essential for biological activities of insulin. A8-10 is not much concerned with biological activities, but is much more important antigenically in binding to its antibodies, these results may help us design a new type of insulin analogue molecule.
- Citation: Yang SZ, Huang YD, Jie XF, Feng YM, Niu JY. Relationship between insulin A chain regions and insulin biological activities. World J Gastroenterol 2000; 6(3): 371-373
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.371
To elucidate the multi-functions of insulin molecule, hundreds of insulin analogues with changes involving 90% of the constituting 51 amino acid residues at various parts of insulin molecule have been prepared by either chemical modification or synthesis during the past two decades. In this communication we report the influences of three different regions of insulin A chain, especially the secondary structure region, i.e. A12-18 helical region, on insulin biological activities. Recently, thorough studies of insulin analogues with deletion of fragments of the molecule were also undertaken, but restricted mostly to changes in the C-terminal part of the B chain[1,2], presumabl y due to its location outside the disulfide linkage and therefore ease of splitting off a tryptic or peptic fragment and recombining with a modified or shortened fragment by chemical or enzymatic semi-synthesis. No such enzymatic site was found in A chain, and most of its amino acid residues are confined inside disulfide linkages. Chemical synthesis might be a better choice for making analogues with modification or deletion in part of the A chain, which constitutes the main feature of this communication.
To obtain insulin analogues, we first set up the semisynthesis strategy of insul in. Insulin A chain was synthesized by the Fmoc solid phase synthesis strategy, and then synthetic A chain S-sulphonate was combined with porcine insulin B chain S-sulphonate forming crystalline insulin[3]. Based on these experiences, the following insulin analogues were semisynthesized. They are [A21Ala]Ins[4], where A21Asn is replaced by Ala, and its derivatives with deletions in the A12-18 helical region, [DesA13-14, A21Ala]Ins[4] and [DesA12-13-14-15, A21Ala]Ins[4], and [A15Asn, A17Pro, A21Al a]Ins, where the helical structure of A12-18 was also demolished by the substitutions of Pro for A17Glu and Asn for A15Gln predicted by Chou-Fasman method, and [A8His, A9Arg, A10Pro, A21Ala]Ins[5], and [A8His, A9Lys, A10Pro, A21Ala]Ins[5], and [A8His, A9Lys, A10Arg, A21Ala]Ins[5], in which His reminiscent A8 of chicken insulin with high biological activity took the place of A8Thr and Arg, Lys, Pro replaced A9Ser and A10Ile to examine the influences of these alterations on receptor binding and antigenicity of insulin. The sequences of insulin A chain analogues are as follows (Figure 1).
Porcine insulin (26.4 U/mg), Shanghai Bioch-emical Plant; Sephade x G-15, G-50, Sepharose CL-6B, Pharmacia; Fmoc amino acid, prepared in the laboratory; DCC, acetonitrile, trimethylsilyl trifluoromethane sulfonate (TMSOTF), thioanisole, Fluka Co. trifluoro-acetic acid (TFA) Merck Co.; DTT, Serva Co.
Peptide synthesis Base labile N-Fmoc protected amino acid was coupled by DCC-HOBt to the 2% cross linked ρ-alkoxybenzyl alcohol resin. The functional side chain groups were protected by tBu for Cys, Glu, Tyr and Ser. Scheme of manipulation was the same as reported[6]. The peptide chain wa s detached from the resin support with simultaneous removal of all tBu protectin g groups by TFA except S-tBu of cysteine. S-tBu was deprotected by M TMSOTf-t hioanisole-TFA system[5] and transformed to S-sulphonate by tetrathion ate and sulfite with the procedure as reported[7].
Isolation and purification of ASSO3- Crude ASSO3- fractions were purified by HPLC with gradient elution (A: 0.1% TFA, B: 60% CH3CN, 0.125% TFA).
Recombination with the natural (porcine) BSSO3- and purification This was carried out according to modified Chance's procedure[4]. The crude product was purified by HPLC using the same procedure as that f or ASSO3-.
Receptor binding, in vivo activities and RIA The receptor -binding of insulin and analogues was determined by the displacement of labelled insulin from the insulin receptor on the human placental membrane according to Feng's[8] procedure. The in vivo activity was estimated semiquantitatively by mouse convulsion assay. RIA kit produced by the Shanghai Biological Product Institute was used for RIA of insulin and analogues.
Insulin A chain analogues were prepared starting from 300-500 mg each of Fmoc alanyl resin and products obtained from each step and the final overall yield is given in Table 1.
| A Chain analogues | FmocAla-resinmg (mmol) | Target peptide-resin (mg) | After HPLC (mg) | Overall yield (%) |
| 1 | 500 (0.19) | 769 | 62.1 | 12.3 |
| 2 | 500 (0.19) | 749 | 54.6 | 12.0 |
| 3 | 500 (0.19) | 724 | 45.4 | 12.4 |
| 4 | 500 (0.32) | 865 | 20.0 | 4.5 |
| 5 | 300 (0.135) | 657 | 39.6 | 10.6 |
| 6 | 300 (0.135) | 648 | 40.1 | 11.2 |
| 7 | 300 (0.135) | 660 | 41.3 | 11.1 |
According to the modified Chance's procedure, insulin analogues were obtained by purification of the crude reconstituted products. The identific ations of amino acid analysis and HPLC indicated homogeneous products of insulin analogues.
The receptor-binding capacities calculated from the receptor binding curve, in vivo activities and immunological activities are shown in Table 2.
| Sample | Receptor binding | Mouse convulsion | Immunological activity |
| Ins | 100 | 100 | 100 |
| 1B | 70.3 | 60 | |
| 2B | 7.9 | 6.2 | |
| 3B | 4.0 | 3.3 | |
| 4B | 10.4 | 10 | |
| 5B | 51.9 | 50 | 28.8 |
| 6B | 44.3 | 40 | 29.6 |
| 7B | 33.1 | 30 | 15.4 |
A21Asn was considered to be a key amino acid of the insulin molecule in regard to its receptor binding. It has been very conservative in the evolutionary process, and when replaced the analogues showed remarkable diminution of biological activity. Our results showed that the analogue with the A21 Asn replaced by Ala exhibited substantial activity in vivo and especially higher level of recept or binding, indicating the non-essentiality of A21Asn in receptor binding and biological activity as well. It could well be replaced by other amino acid residues with good retention of biological activities. Better results were observed when the side chain of the A21 amino acid is smaller or not present (Gly)[9]. However, deletion of the A21 residue is fatal as the activity of desA21 insulin showed only less than 1% of the activity. Apparently, like some non-essential amino acid residues in the insulin molecule, A21 is important in maintaining the specified spatial configuration of insulin required for its biological activities.
It is generally accepted that during the reconstitution of A and B chain to insulin A6-11 disulfide bond formed first and this led to the formation of right natural conformation by self adjustment. When the B chain was getting in touch wit h the intrachain disulfide, A6-11, a hydrophobic nucleus was formed to enhance the proper pairing of two Cys in both ends of the B chain to corresponding Cys of the A chain[10]. The success of our reconstitution with deleted A chain supports to a certain extent this suggestion. It showed that the C-terminal helical region in the A chain of insulin was not as important as the N-terminal helical region in reconstitution with the B chain. The biological activities of insulin analogues with gradually damaged structural integrity in A12-18 helical region were similar to that of insulin analogue with the demolition of the A12 -18 helical region by the substitution of Pro and Asn for A17Glu and A15Gln respectively. The observations suggested that the helical structure of A12-18 is essential for biological activities of insulin.
It was generally suggested that the enhanced activities of [A8His]Ins[11], chicken and turkey insulins were ascribed to the His at A8[12-14], which should account for the higher affinity for insulin receptor. Our results showed that the presence of His at A8 apparently did not enhance the receptor binding. The receptor binding of [A8His]Ins should lie at the range of about 50%. The enhanced potency of these insulins containing His at A8 may not result solely from an apparent and direct effect due to the presence of A8His. A8His and A10Arg signify a higher influence on insulin antigenicity. A8His will enhance the binding activity to the anti-insulin serum[11], while A10Arg will decrease this binding, but amino acid at A9 had very little to do with antigenic activity. In addition to studies on how to elucidate the action of an insulin molecule to its receptor and express the physiological activity that follows such action, the purpose of studies on the structure activity relationship of in sulin will rely more on how to get a better analogue with high potency but low antigenicity. We hope that the information provided in this study will be beneficial to the development of a new type insulin analogue with those qualities through design of an appropriate architecture of this important hormone.
Dr. Shi-Zhen Yang, graduated from Beijing Scientific and Technical College, Academia Sinica, in 1964, associate researcher, majoring in peptide synthesis, having 40 papers published.
Edited by You DY and Zhu LH
proofread by Sun SM
| 1. | Shanghai Insulin Research Group. Structural studies on despentapeptide (B26-30)insulin. Zhongguo Kexue. 1976;4:351-356. |
| 2. | Chen LT, Huang CM, Zhang M, Yang ZR, Hu MH. Purification and characterization of Des-terpeptide of C-terminal (B28-30) human insulin. Shengwu Huaxue Zazhi. 1996;12:187-195. |
| 3. | Yang SZ, Niu JY. Solid phase synthesis of the A-chain of insulin and its recombination with the B-chain to crystalline insulin. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 1992;24:497-502. |
| 4. | Yang SZ, Huang YD, Feng YM, Niu JY. Insulin analogues with deletions at the helical region of the A chain. Shengwu Huaxue Yu ShengwuWuli Xuebao. 1994;26:49-55. |
| 5. | Yang SZ, Lin W, Huang YD, Feng YM, Niu JY. Insulin analogues with alteration at A8-10. Acta Biochemi Biophys Sin. 1995;27:329-334. |
| 6. | Yang SZ, Niu JY. Solid phase synthesis of serum thymic factor and preparation of its antiserum. Shengwu Huaxue Zazhi. 1991;7:349-354. |
| 7. | Paynovich RC, Carpenter FH. Oxidation of the sulfhydryl forms of insulin A-chain and B-chain. Int J Pept Protein Res. 1979;13:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Feng YM, Zhu JH, Zhang XT, Zhang YS. Studies on the mechanism of insulin action. Acta Biochimi Biophys Sin. 1982;14:137-143. |
| 9. | Markussen J, Diers I, Hougaard P, Langkjaer L, Norris K, Snel L, Sørensen AR, Sørensen E, Voigt HO. Soluble, prolonged-acting insulin derivatives. III. Degree of protraction, crystallizability and chemical stability of insulins substituted in positions A21, B13, B23, B27 and B30. Protein Eng. 1988;2:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Peking Insulin Structure Research Group. Studies on the insulin crystal structure: the molecule at 1.8A resolution. Zhongguo Kexue. 1974;2:752-761. |
| 11. | Märki F, de Gasparo M, Eisler K, Kamber B, Riniker B, Rittel W, Sieber P. Synthesis and biological activity of seventeen analogues of human insulin. Hoppe Seylers Z Physiol Chem. 1979;360:1619-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Pullen RA, Lindsay DG, Wood SP, Tickle IJ, Blundell TL, Wollmer A, Krail G, Brandenburg D, Zahn H, Gliemann J. Receptor-binding region of insulin. Nature. 1976;259:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 275] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Weitzel G, Renner R, Kemmler W, Rager K. [Structure and increased activity of insulin from the turkey (Meleagris gallopavo)]. Hoppe Seylers Z Physiol Chem. 1972;353:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Simon J, Freychet P, Rosselin G. Chicken insulin: radioimmunological characterization and enhanced activity in rat fat cells and liver plasma membranes. Endocrinology. 1974;95:1439-1449. [PubMed] |