Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.361
Revised: February 3, 2000
Accepted: February 28, 2000
Published online: June 15, 2000
AIM: To investigate the mechanisms of salvianolic acid A (SA-A) against liver fibrosis in vitro.
METHODS: NIH/3T3 fibroblasts were cultured routinely, and incubated with 10-4 mol/L-10-7 mol/L SA-A for 22 h. The cell viability was assayed by [3H]proline incorporation, cell proliferation by [3H]TdR incorporation, cell collagen synthetic rate was measured with [3H]proline impulse and collagenase digestion method. The total RNA was prepared from the control cells and the drug treated cells respectively, and α (1) I pro-collagen mRNA expression was semi-quantitatively analyzed with RT-PCR.
RESULTS: 10-4 mol/L SA-A decreased cell viability and exerted some cytotoxiciy, while 10-5 mol/L-10-7 mol/L SA-A did not affect cell viability, but inhibited cell proliferation significantly, and 10-6 mol/L SA-A had the best effect on cell viability among these concentrations of drugs. 10-5 mol/L-10-6 mol/L SA-A inhibited intracellular collagen synthetic rate, but no significant influence on extracellular collagen secretion. Both 10-5 mol/L and 10-6 mol/L SA-A could decrease α (1) I pro-collagen mRNA expression remarkably.
CONCLUSION: SA-A had potent action against liver fibrosis. It inhibited NIH/3T3 fibroblast proliferation, intracellular collagen synthetic rate and type I pro-collagen gene expression, which may be one of the main mechanisms of the drug.
- Citation: Liu CH, Hu YY, Wang XL, Xu LM, Liu P. Effects of salvianolic acid-A on NIH/3T3 fibroblast proliferation, collagen synthesis and gene expression. World J Gastroenterol 2000; 6(3): 361-364
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.361
Radix salviae miltiorrhizae, one of the most frequently used Chinese herbs, is regarded to have effects on both blood production and circulation by traditional Chinese medicine, and is widely applied in clinical therapy for liver diseases, such as chronic hepatitis, hepatic cirrhosis, etc. Salvianolic Acid-A is one of the water soluble components from Radix salviae miltiorrhizae. It was reported to have good actions on peroxidation[1]. Lipid peroxidation could stimulate hepatic stellate cell (HSC) transformed into myofibro blast like cell (MFBC) and collagen gene expression in vivo and in vitro, and played an important role in liver fibrogenesis[2]. In our previous work[3], it was found that SA-A could protect hepatic lipid peroxidation, and had marked effects against liver injur y and fibrosis in carbon tetrachloride induced fibrotic rats. In order to investigate the mechanism by which SA-A protects against liver fibrosis, we observed the effects of SA-A on NIH/3T3 fibroblast proliferation, collagen protein production and procollagen gene expression.
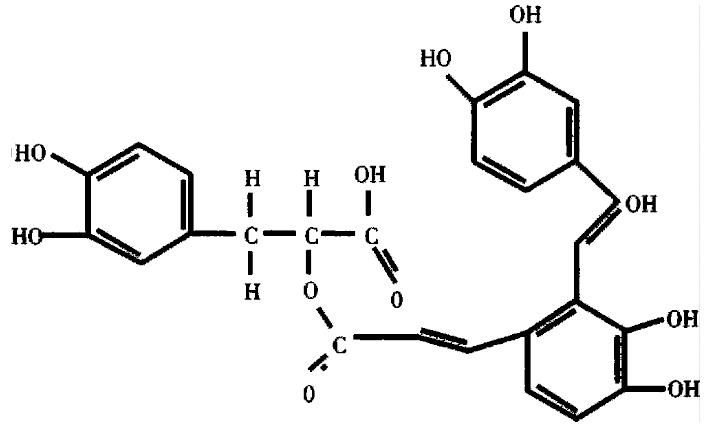
SA-A, molecular formular as C26H22O10, molecular structure as shown in Figure 1, molecular weight 494, was extracted and identified by Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
PRMI-1640 Medium and Dubocal modified Eagle Medium (DMEM) were purchased from Gibco BRL Co., new brown serum (NBS) from Shanghai Sino-American Co., purified type III collagenase (specific activity, 960 U/mg), N-ethylmaleimide (NEM) and β-aminopropionitrile from Sigma Co. [53H]proline ([3H]Pro) from Amersham Co. methyl-[3H] thymidine (TdR) from Shanghai Institute of Atomic Energy, guanidium thiocynate from Serva Co. Access RT-PCR Sy stem Kit, PCR marker from Promega Co., Diethypyrocarbonate, saturated phenol/chloroform mix and agarose from Shanghai Sangon Biotech Co. Other reagents all were of analytical grade.
The non-homogeneous scintillation liquid was dimethylbenzene solution containin g 5 g/L 2,5-diphenyloxazol (PPO) and 0.5 g/L 1,4-bis[5-phen yloxazol-2] benzene (POPOP), the homogeneous scintillation liquid was dimethy lbenzene solution containing 7 g/L PPO, 0.5 g/L POPOP, 100 g/L naphthalene and 400 mL/L2-ethoxy-ethanol.
Mouse NIH/3T3 fibroblasts were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, and cultured with PRMI-1640 medium containing 100 g/L NBS, 100 KU/L penicillin and 100 mg/L streptomycin. After the cell growth became confluent, they were digested with trypsin-EDTA and subcultured.
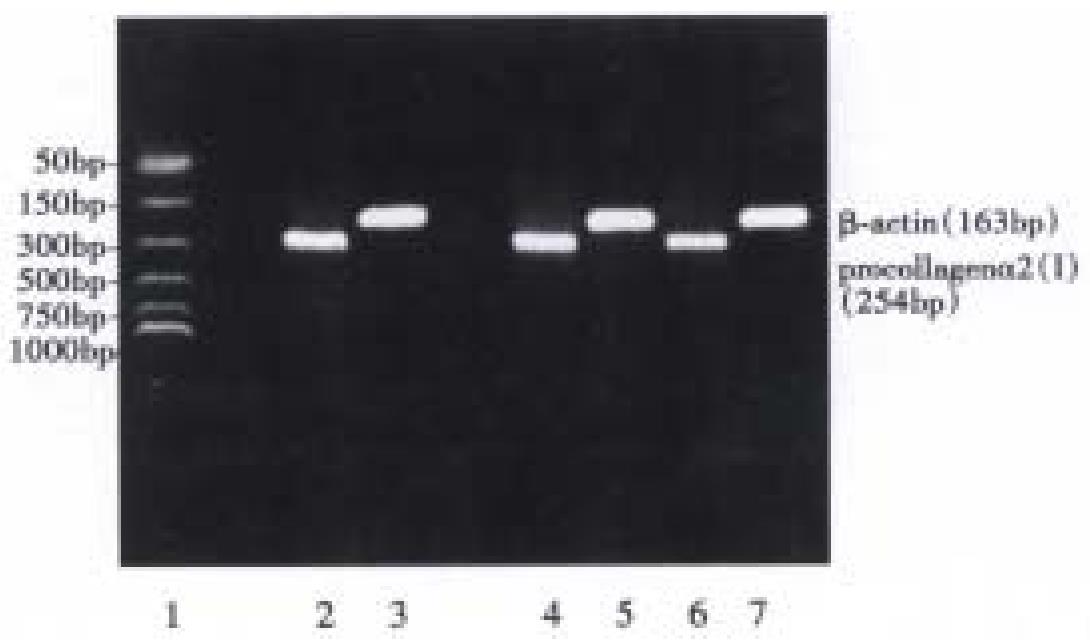
The PCR primers for pro-collagen α2(I) and β-actin were designed according to the published sequences and references in Table 1[4], and were synthesized by Gibco BRL Co.
| Primers | Sequence | Size |
| α 2(I) collagen upstream | 5'TGTTCGTGGTTCTCAGGGTAG3' | |
| α 2(I) collagen downstream | 5'TTGTCGTAGCAGGGTTCTTTC3' | 254 bp |
| β-actin upstream | 5'ACATCTGCTGGAAGGTGGAC3' | |
| β-actin downstream | 5'GGTACCACCATGTACCCAGG3' | 163 bp |
Confluent NIH/3T3 fibroblasts in 24 well plates were incubated with 10-4 mol/L-10-7 mol/L SA-A diluted in PRMI-1640 medium containing 100 mL/L NBS for 22 h, and [3H]TdR (55.5 KBq/well) was impulsed in the last 16 h. Then cells were harvested with trypsin digestion an d collected on the filtration membrane, then sample radioactivity (cpm) in the non-homogeneous scintillation liquid was measured by Backman Wallac 1410 Scintillator. All tests were repeated 3 times.
According to Mallat's method[5], confluent NIH/3T3 fibroblast s in 24-well plates were incubated with 10-4 mol/L-10-7 mol/L SA-A re solved in PRMI-1640 medium without NBS for 22 h, and [3H]Pro (55.5 KB q/well) was impulsed in the last 16 h. Then cells were collected and the cpm was measured as above.
According to Greets' method[6], confluent NIH/3T3 fibroblasts in 6 well plates were incubated with 10-5 mol/L-10-6 mol/L SA-A diluted in PRMI-1640 without NBS for 22 h, during the later 16 h the culture media were changed to DMEM containing 185 KBq/mL [3H]Pro, 100 mg/L-β-aminopropionitrile, 50 mg/L ascorbic acid as well as the same drugs. Then the culture media and cell layer extract were collected respectively, dialyzed thoroughly and reacted with collagenase, etc. The total radioactivity in the samples (cpmt), radioactivity in the samples treated with colla genase (cpmc) and not treated with collagenase (cpmb) were counted in the ho mogeneous scintillation liquid by Backman Wallac 1410 Scintillator. The new collagen that cell produced, i.e. the fraction of collagenous protein express edas percentage of total radiolabeled protein, was calculated using the formula:
% of collagen = 100 ÷ [5.4 × (cpmt - cpmc)/(cpmc - cpmb) + 1]
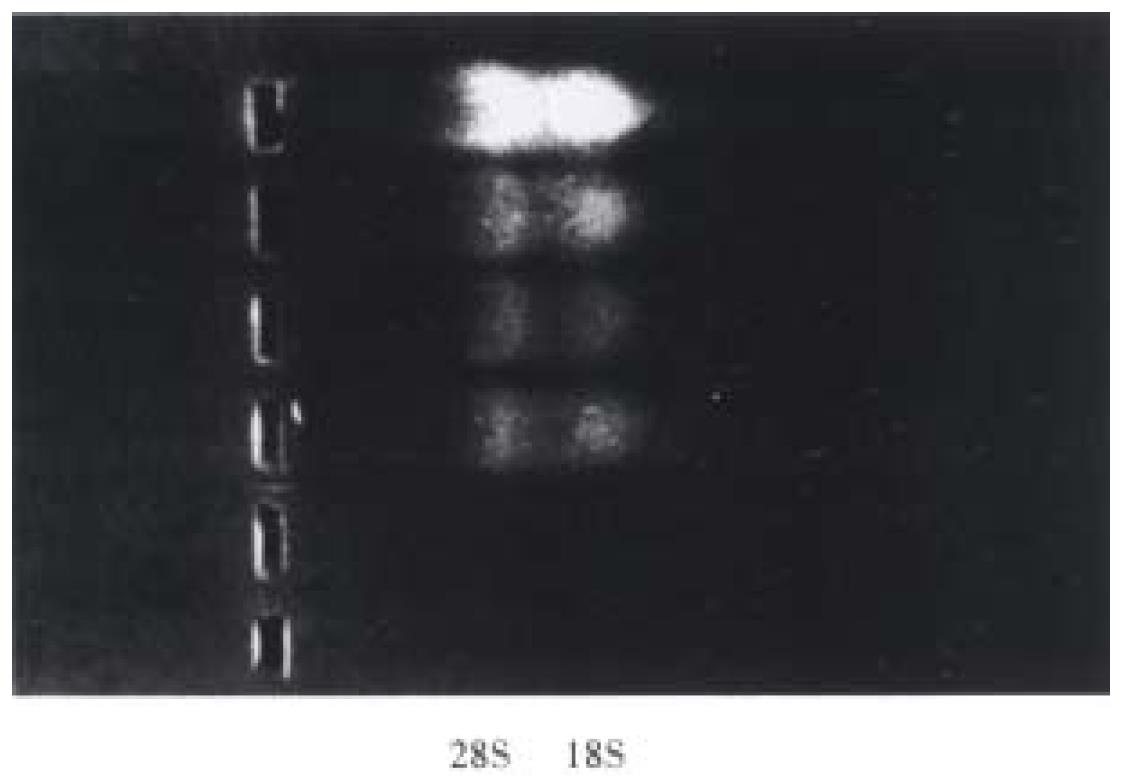
The total RNA was extracted from the control cells and SA-A incubated cells by the acid guanidium thiocynate-phenol-chloroform method[7]. The RNA quantity was determined by absorption at 260 nm, its purity was confirmed with A260/A280 specrophoto meter readings that ranged from 1.6 to 1.9, and its integrity was checked by 9 g/L agarose gel electrophoresis with ethidium bromide (EB) staining of 18S and 28S ribosomal RNA (Figure 2). With Access RT-PCR system kit, the cDNA synthesis and amplification was done in one tube following the manufacturer's instructions. In brief, 1 μg RNA, 50 pmol/L primers for α (1) I pro-collagen or β-actin were added to each reaction mixture respectively, which included 10 mmol/L dNTPs 1 μL, 25 mmol/L MgSO4 2 μL, AMV reverse transcriptase 5 U, Tfl DNA polymerase 5 U, AMV/Tfl-5 × buffer 10 μL. The reaction final volume was 50 μL and was covered with 20 μL mineral oil. Then with PCR Touchdown thermal cycler (Hybaid, England), RT-PCR reaction was run in the following procedures: ① 48 °C for 45 min, 1 circle. ② 94 °C for 2 min, 1 circle. ③ 94 °C for 30 s, 60 °C for 1 min, 38 °C for 2 min, 30 circles. ④ 68 °C for 7 min, 1 circle. Five μL PCR product was run on 15 g/L agarose gel and observed by EB staining under UV light, the electrophores is photo was transformed into computer, and α 1(I) pro-collagen intensity was analyzed with MPIAS500 image system, while the β-actin band intensity was subtracted as an internal standard.
Data were analyzed by Student's t test.
10-5 mol/L-10-7 mol/L SA-A had no marked effects on cell morphology, but 10-4 mol/L SA-A led to shrinkage and detachment of some cells, showing cytotoxicity to some degree. 10-4 mol/L-10-7 mol/L SA-A did not decrease intercellular [3H]Pro incorporation, while 10-6 mol/L SA-A could increase [3H]Pro impulse(P < 0.05) and enhance cell viability (Table 2).
10-4 mol/L-10-6 mol/L SA-A remarkably decreased intercellular [3H]TdR incorporation and inhibited cell proliferation(P < 0.05), 10-4 mol/L SA-A showed more significant effect (P < 0.01), but it induced some cell death, which may be associated with its cytotoxic action. 10-7 mol/L SA-A had no obvious effect on cell [3H]TdR incorporation (Table 2).
10-5 mol/L-10-6 mol/L SA-A could inhibit intracellular collagen synthetic rate significantly (P < 0.01), but did not influence extracellular synthetic rate (Table 3).
Both 10-5 mol/L and 10-6 mol/L SA-A decreased procollagen α1(I) mRNA expression significantly (P < 0.05), but the re was no difference between the two different concentration groups (Table 4, Figure 3).
Hepatic fibrosis, a precursor of cirrhosis, is a common and important pathologic al feature of chronic liver diseases, which involves the abnormal accumulation of extracellular matrix (ECM) proteins, particularly collagen[8]. In fibrotic liver, ECM components are mainly produced by HSC and fibroblasts. It is known that during fibrogenesis, HSC undergoes a process of activation, developing a myofibroblast-like phenotype associated with increased proliferation and ECM production, especially type I collagen synthesis. The mouse NIH/3T3 fibroblast also shared the features that active HSC (MFBC) presented, such as remarkable proliferation and substantial production of collagen, and stable cell line. In practice, NIH/3T3 fibroblast is often used as a desirable cell model for investigation of antifibrotic drugs.
In order to rule out the possibility of SA-A cytotoxic influence in vitro, the intracellular [3H] Pro incorporation was measured, and inverted microscopic observation was done. It was found that only 10-4 mol/L SA-A cause d some cell detachment, decreased [3H] Pro incorporation, and showed cytotoxicity to some extents. 10-5 mol/L-10-7 mol/L SA-A did not influence cell morphology or inhibit cell viability. However, 10-6 mol/L SA-A enhanced cell viability. Both 10-5 mol/L-10-6 mol/L SA-A could inhibit intracellular [3H]TdR impulse that NBS stimulated. It is suggested that SA-A had an effective action against NIH/3T3 fibroblast proliferation.
Type I collagen is the predominant component of ECM during liver fibrosis. Its production involve two processes: the first is intracellular synthesis, including gene transcription, translation and modification to form procollagen, then procollagen alpha chains are secreted to the outside of the cell to form helix collagen by sorting and alignment etc. In the study, it was found that SA-A d ownregulated procollagen α2(I) steady-state mRNA expression, and intracellular collagen synthetic rate, but exerted no effect on extracellula r synthetic rate. It is suggested that SA-A influence on collagen production through the intracellular synthetic process. The fibrogenic cells have two predominant features: one is active in cell proliferation, which led to increase in c ell number, another is strong fibrogenic ability per cell, which led to accumulation of ECM. In the study, SA-A not only inhibited NIH/3T3 fibroblast proliferation, but also decreased collagen synthesis, showing a good action against liver fibrosis.
Salvianolic radix is widely used as an important component in Chinese herbal formulas for the treatment of chronic liver diseases. Salvianolic Acid-A, one of water-soluble ingredients from Salvianolic radix, had effective actions on hepatic peroxidation and fibrosis in vivo[3]. In the paper, it is for the first time found that SA-A has the potential action against hepatic fibrosis in vitro, and its main mechanisms of antifibrotic action perhaps was associated with the inhibition of fibrogenic cell proliferation, collagen gene expression and protein synthesis.
Dr. Cheng-Hai Liu, graduated from Shanghai University of Traditional Chinese Medicine as PhD in 1996, associate professor, majoring in hepatology, having 20 papers and 4 books published.
Edited by Zhu LH
proofread by Sun SM
| 1. | Lin TJ, Liu GT. Protective effect of salvianolic acid A on heart and liver mitochondria injury induced by oxygen radicals in rats. Zhongguo Yaolixue Yu Duli Xuebao. 1991;5:276-281. |
| 2. | Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836-847. [PubMed] |
| 3. | Hu YY, Liu P, Liu C, Xu LM, Liu CH, Zhu DY, Huang MF. Actions of salvianolic aicd A on CCl4 poisoned liver injury and fibrosis in rats. Zhongguo Yaoli Xuebao. 1997;18:478-480. |
| 4. | Mallat A, Preaux AM, Blazejewski S, Rosenbaum J, Dhumeaux D, Mavier P. Interferon alfa and gamma inhibit proliferation and collagen synthesis of human Ito cells in culture. Hepatology. 1995;21:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Power WJ, Kaufman AH, Merayo-Lloves J, Arrunategui-Correa V, Foster CS. Expression of collagens I, III, IV and V mRNA in excimer wounded rat cornea: analysis by semi-quantitative PCR. Curr Eye Res. 1995;14:879-886. [PubMed] |
| 6. | Geerts A, Vrijsen R, Rauterberg J, Burt A, Schellinck P, Wisse E. In vitro differentiation of fat-storing cells parallels marked increase of collagen synthesis and secretion. J Hepatol. 1989;9:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] |
| 8. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |