Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.348
Revised: April 3, 2000
Accepted: April 28, 2000
Published online: June 15, 2000
AIM: To directly radiolabel an anti-hepatoma mAb fragment HAb18 F(ab')2 with 99mTc by stannous-reduced method, and assess the stability, biodistribution and radioimmun oimaging (R II).
METHODS: Immunoreactive fraction was determined according to Lin dmo's method. Ellman's reagent was used to determine the number of thiols in the reduced F(ab')2. Labeling efficiency and homogeneity were measured by paper chromatography, sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) and autora diography. Challenge assay involved the incubation of aliquots of labeled antibody in ethylenediaminetetraacetate (EDTA) and L-cysteine (L-cys) solutions with different molar ratio at 37 °C for 1 h, respectively. Investigations in vivo utilized nude mice bearing human hepatocellular carcinoma (HHCC) xenografts with gamma camera imaging and tissue biodistribution studies at regular intervals.
RESULTS: The labeling procedure was finished within 1.5 h compared with the "pretinning" method which would take at least 21 h. In vitro studies demonstrated that the radiolabeled mAb fragment was homogen eous and retained its immunoreactivity. Challenge studies indicated that 99mTc-labeled HAb18 F(ab')2 in EDTA is more stable than in L-cys. Imaging and biodistribution showed a significant tumor uptake at 24 h post-injection of 99mTc-labeled HAb18 F(ab')2. The blood, kidney, liver and tumor uptakes at 24 h were 0.56 ± 0.09, 56.45 ± 11.36, 1.43 ± 0.27 and 6.57 ± 3.01 (%ID/g), respectively.
CONCLUSION: 99mTc-HAb18 F(ab')2 conjugate prepare d by this direct method appears to be an effective way to detect hepatoma in nude mice model.
- Citation: Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment: a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol 2000; 6(3): 348-352
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.348
The introduction of mAbs as targeting devices in nuclear medicine is well developed and many different antibodies which labeled with a variety of isotopes have been reported in cancer diagnosis. It seemed that 99mTc is the most popular radionuclide for nuclear medicine imaging because of its favorable physical characteristics, low cost, and ready availability. 99mTc labeled mAb fragments should be superior to other big molecule radioimmunoconjugates for u se in tumor R II. A number of methods have been proposed for 99mTc labeling proteins, and mAbs in particular. In general, these methodologies can be divided into two categories: indirect and direct methods[1]. In indirect method the protein was modified with a technetium binding ligand and then reacted with a technetium complex. Several bifunctional chelating agents have been syn thesized and used, such as diethylenetriaminepenta acetic acid (DTPA)[2], diamide dimercaptide N2S2 ligands, and hydrazino nicotinamide analog[3]. Although it is said that the indirect method can lead to loss of immuno reactivity. Joiris et al[4] have tested that the derivatization of antibody or fragment by iminothiolane does not split the protein and keeps the immunoreactivity. By direct method, 99mTc metal ion binds directly to endogenous donor groups on the antibody. The method is simple to perform and compatible with practical clinical use. However, direct labeling of mAbs with 99mTc was reported to be unstable due to non-specific binding (low and high-affinity)[5,6], but some reports suggest an improved labeling of proteins with 99mTc. In the Schwarz and Steinstrasser procedure, as modified by Mather and Ellison[7], disulide bridges in the mAb are reduced with 2-mercaptoethanol (2-ME). After purification, the resulting reduced antibody can be stored frozen until required for use. Labeling is accomplished by addition of stannous ion from a bone- scanning kit and pertechnetate. In addition to using regular reducing agents, such as 2-ME, stannous ions[8], borohydride[9], ascorbic acid[10], dithionite[11], or glutathione[12]to generate sulphydryl groups, other peculiar approaches also appeared recently. Direct 99mTc labeling of mAbs were finished by reduction of antibodies using photoactivation and insoluble macromolecular Sn(II) complex[13,14]. With the development of direct method, there have been a few reports of successful use of this technique in colorectal, breast, and ovarian cancer imaging[15-17].
In this report, we describe a direct method for radiolabeling anti-hepatoma monoclonal antibody fragment HAb18 F(ab')2 with 99mTc. The stability and homogeneity of 99mTc-HAb18 F(ab')2 were evaluated. The biodis tribution and tumor localization in nude mice bearing a HHCC xenograft were studied.
The mAb HAb18 is of murine IgG1 isotype and was developed by our laboratory[18]. F(ab')2 fragment of HAb18 was generated by papain digestion with a molecular weight of ~96000 dalton[19].
Hepatocellular carcinoma grown in Balb/c mice was used as a prototype tumor model. Approximately 107 HHCC cells obtained from Shanghai Cell Institute of Chinese Academy of Sciences were implanted in the left thigh of the animals and the tumors were allowed to grow for 8-10 d to approximately 1 cm in diameter.
The antibody concentrated to 8 g/L in neutral PBS was reduced by reaction with a molar excess of stannous/glucoheptonate (Sn/GH) ranging from 10:1 to 50:1 (Sn/GH: MAb) at 37 °C for 15-30 min. The Sn/GH with a mass ratio of 1:100 was dissolved in 50 mM acetate-buffered saline (ABS), pH5.3 purged with nitrogen. The reduced antibody was isolated from reductant t hro ugh a PD-10 column (Pharmacia) equilibrated with 0.05 mol/L ABS. The number of resulting free sulphydryl groups was assayed with Ellman's reagent 5, 5'dithio-bis (2-nitrobenzoic acid), (DTNB, Sigma Chemical Co., USA)[20]. One hundred μL of sample was mixed with 20 μL of 0.01 mol/L-DTNB and diluted to 3 mL with 0.05 mol/L Tris-HCl buffer pH8.4. The mixture was incubated at room te mperature for 15 min and coloration measured with an UV/VIS spectrophotome ter at 412 nm. The number of thiols was obtained by comparison with a series of L-cysteine (L-cys) standards ranging from 0.312 mg/L to 10 mg/L.
The integrity of the reduced F(ab')2 was determined by non-reduced SDS-PAGE with 100 g/L gel using Vertical Gel Electrophoresis System (Bio-Rad). The gel was stained with Coomassie brilliant blue R250. Control experiments were run using unreduced mAb F(ab')2.
For labeling, 160 μg of reduced HAb18 F(ab')2 was mixed with a 10 μL-20 μL of diluted Sn/GH solution (0.2 g/L), and pertechnetium solution (0.2 mL, 74 MBq), (Chinese Academy of Atomic Energy) was injected into the mixture. The Sn/GH solution was freshly prepared each time by dissolving 100 mg GH and 1 mg SnCl2·2H2O in 5 mL of saline purged with nitrogen. The reaction mixture was incubated for 0.5-1 h at 37 °C before it was analyzed by Whatman 3 MM paper chromatography which was then developed in acetone or 100 g/L trichloroacetic acid (TCA). R-f values for acetone are: mAb 0.0, 99mTc-GH 0.0, and 99mTcO4-0.9-1.0. R-f values for 100 g/L TCA are: mAb 0.0, 99mTcGH 0, and 99mTcO4- 0.7. Labeled mAb was differentiated from 99mTc colloid by the method of Thrall et al[21]. The same strips impregnated with 10 g/L-20 g/L human serum albumin before development with 5:2:1, water:ethanol:5N NH4OH. Colloid remained on the bottom of the strip while mAb-bound isotope migrated with the solvent front.
The integrity of the labeled F(ab')2 was assayed using the same non-reduced SDS-PAGE as described above. The gel was autoradiograghied on x-ray film before stained with Coomassie brilliant blue R250.
The in vitro immunoreactivity of the radiolabeled HAb18 F(ab')2 was evaluated by a live cell assay[22]. Briefly, HHCC cells 5 × 109/L were centrifuged (1000 r/min) for 5 min and washed twice with 1% bovine serum albumin (BSA) in PBS, then 5 serial 1:2 dilutions were made in 10 g/L BSA in Eppendorf tubes precoated with BSA. Radiolabeled HAb18 F(ab')2 at a concentration of 40 ng/mL in 10 g/L BSA was added using a volume equal to half the volume of cell suspension. The total volume of cell-binding assay solution was 0.3 mL. After incubation for 2 h at 37 °C, the total as well as the cell-bound radioactivity were counted in a gamma counter.
The stability was analyzed by using two different challenging agents, EDTA and L-cys. An aliquot of 50 μL 99mTc-HAb 18 F(ab')2 solution was incubated with EDTA or L-cys at 37 °C for 1 h. The molar ratio of mAb to challenging agent was at a maximum of 10000:1. Dissociation ratio was analyzed on paper chromatography.
Balb/c mice bearing HHCC were divided into three groups. Each group consisted of three animals and each animal received approximately 15 μg antibody with about 7.4 MBq through a lateral tail vein. At time intervals of 4, 10 and 24 h postinjection, three groups of mice were killed, and imaged on a SPECT (Starcam 3000, UK). Data were collected 100000 counts per image and peak energy settings at the 140 keV (20%) window for 99mTc. The blood and other organs of interest were collected. Tissues were washed, blotted, weighed and counted in a gamma counter. For each mouse, data are expressed as percent of injected dose per gram of tissue (%ID/g) after physical decay corrected.
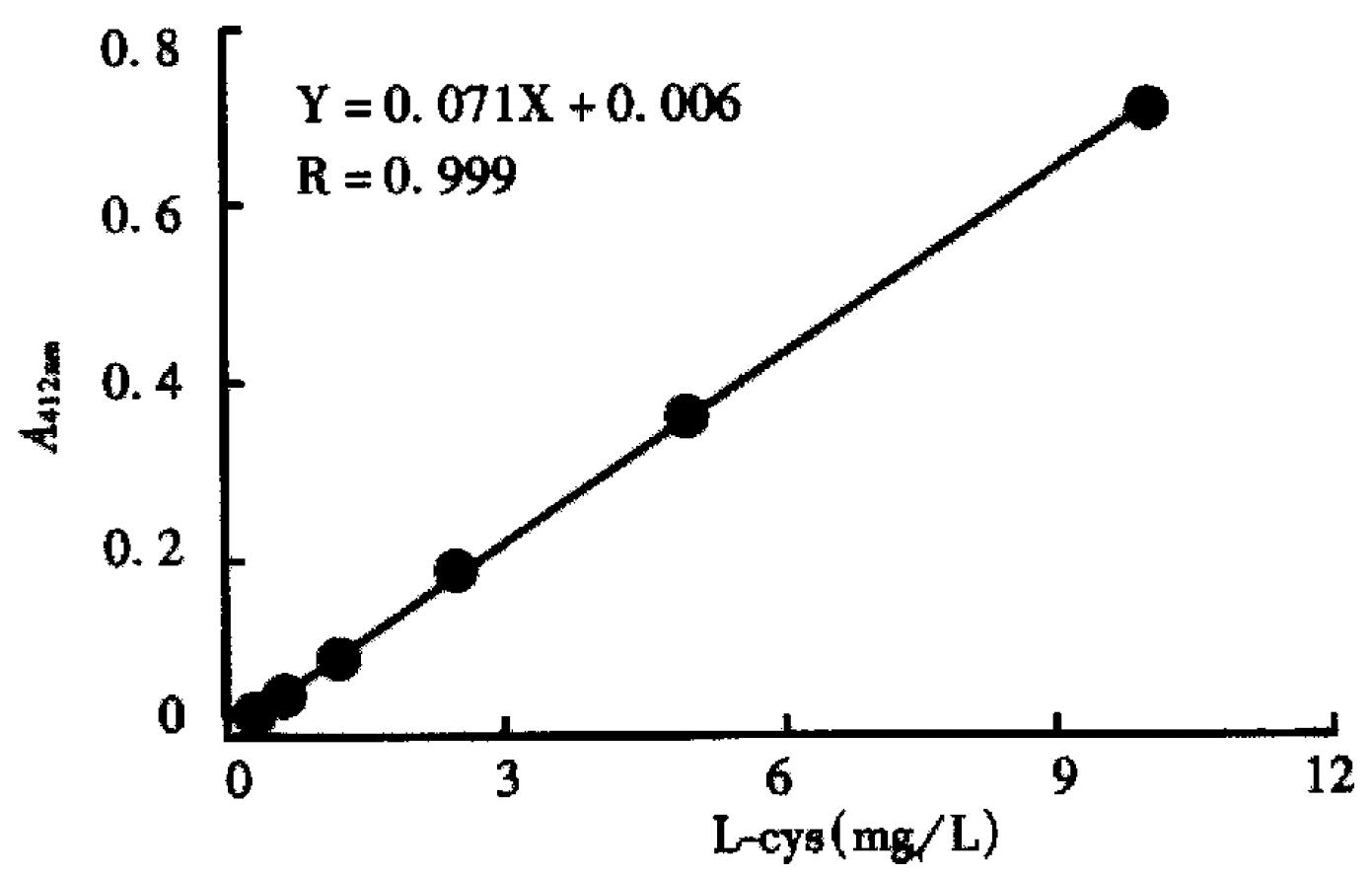
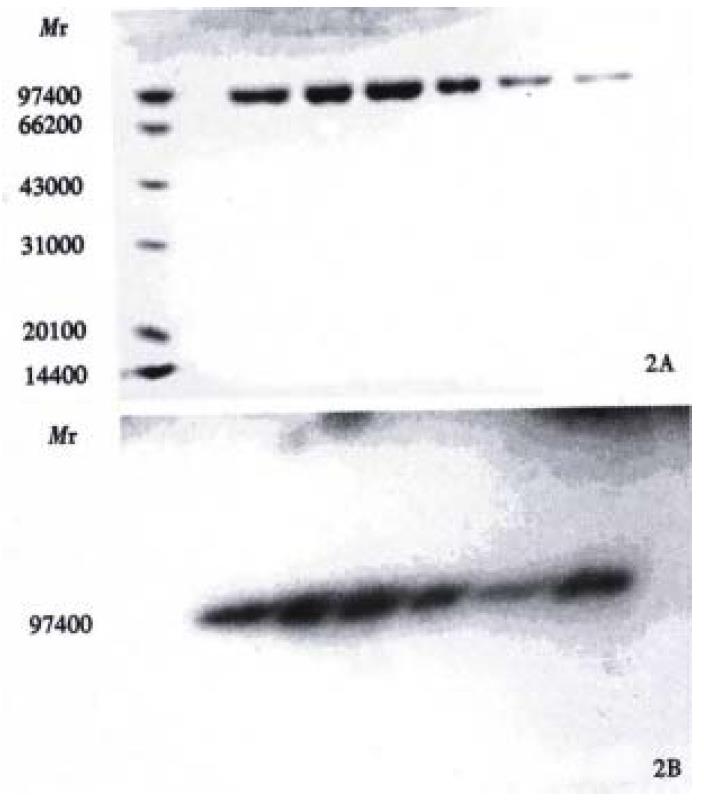
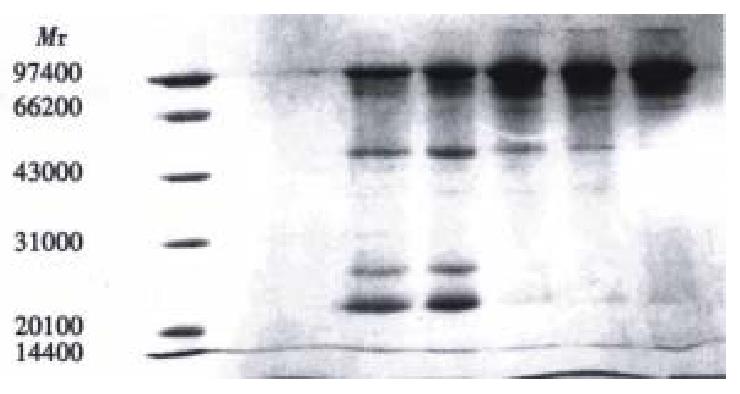
Figure 1 represents the calibration curve for the determination of sulphydryl groups using L-cys standards over a range of 0.312 to 10 mg/L, by plotting optical density at 412 nm versus L-cys standard concentrations after subtraction of the background due to Ellman's reagent. Linear regression was used and correlation coefficient 0.999 was obtained. Table 1 shows the influence of the reduction conditions on the number of free sulphydryl groups detected by this thiol assay. As expected, increasing the molar ratio of Sn/GH to antibody in the reaction mixture does increase the number of apparent-SH groups per antibody, and increase the labeling efficiency correspondingly, which results in the labeling efficiency at a maximum of 84.2%. The free 99mTcO4- and colloid amounts determined by Whatman 3 MM paper using different developing systems were also showed in Table 1. In control experiments, labeling efficiency was 2% when unreduced HAb18 F(ab')2 was used. SD S-PAGE by both staining and autoradiography showed that the radioactivity co-m igrated with the proteins and that there were almost no protein fragments prese nt within the 60:1 of molar ratio of Sn/GH to mAb (Figure 2). However, another SDS-PAGE in Figure 3 illustrates that fragmentation occurred during t he reduction procedure when the molar ratio of Sn/GH to mAb was at 500:1.
| Molar ratio(Sn/GH: mAb) | SH groups/mAb | 99mTcO4- | colloid | labeling efficiency |
| Control | 0 ± 0 | 62.1 ± 4.5 | 1.1 ± 1.2 | 2.0 ± 0.9 |
| 10:1 | 0.43 ± 0.04 | 3.0 ± 1.4 | 1.4 ± 1.1 | 44.6 ± 3.8 |
| 20:1 | 1.25 ± 0.10 | 2.9 ± 1.1 | 1.4 ± 0.7 | 72.8 ± 5.1 |
| 30:1 | 2.46 ± 0.08 | 2.0 ± 0.9 | 3.2 ± 1.4 | 78.6 ± 3.2 |
| 40:1 | 3.34 ± 0.09 | 1.8 ± 1.2 | 2.8 ± 1.3 | 84.2 ± 2.8 |
| 50:1 | 3.61 ± 0.12 | 2.1 ± 0.8 | 3.6 ± 1.5 | 84.4 ± 3.4 |
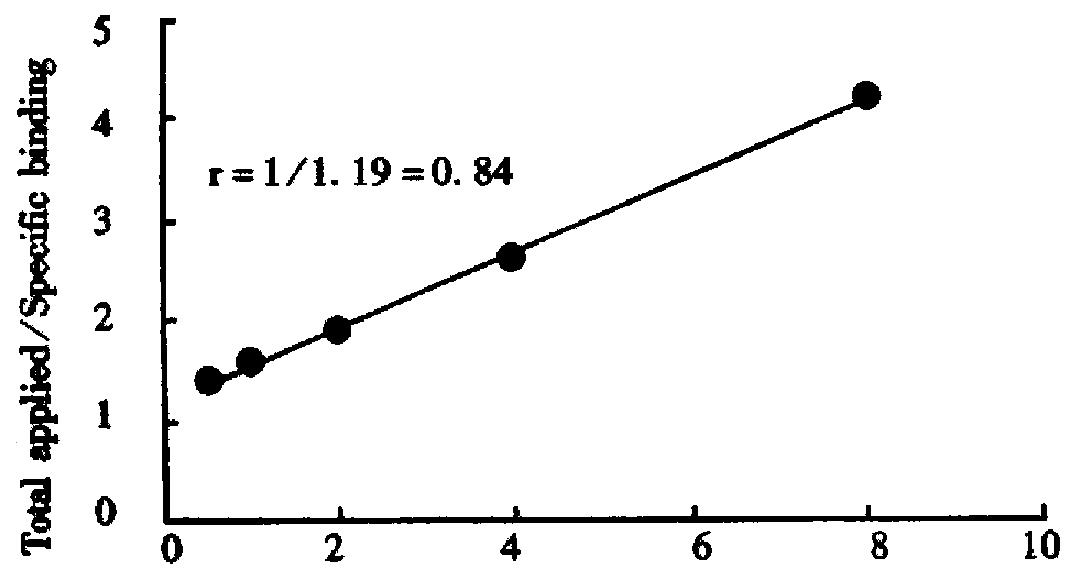
As shown in Figure 4, the immunoreactive fraction, 0.84 was determined by plotting the inverse of the bound fraction compared with the inverse of the cell concentration, which is based on the assumption that the total antigen concentration (cell concentration) is a good enough approximation for the free antigen concentration.
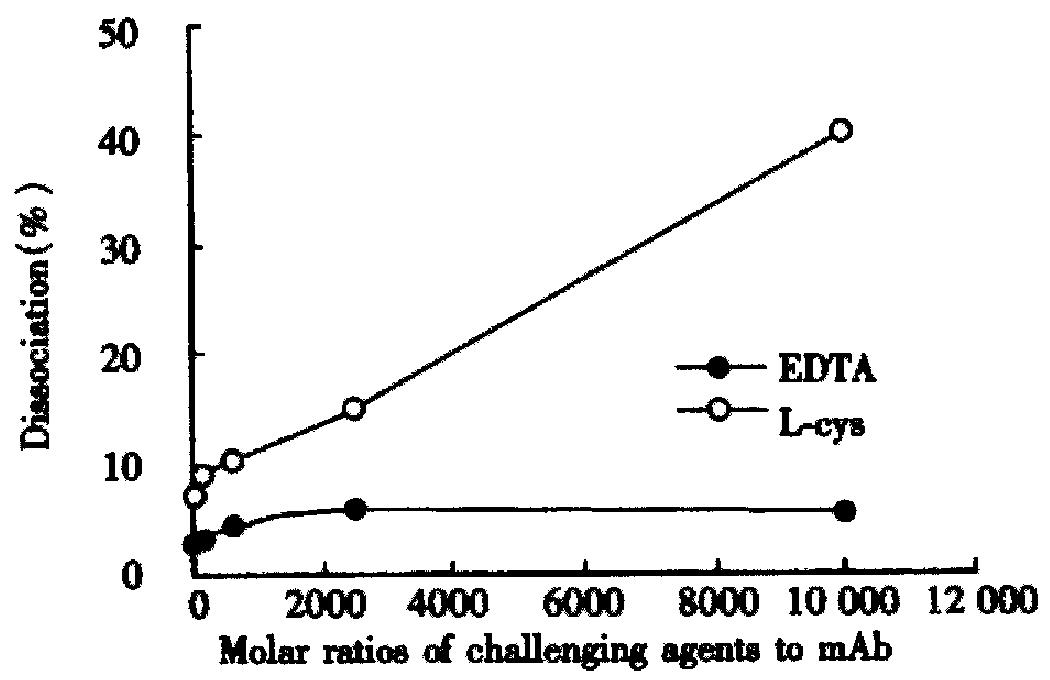
Challenging with EDTA did not remove 99mTc from the labeling conjugate remarkably, while L-cys at a molar ratio of 625:1 remove approximately one-tenth of the label (Figure 5).
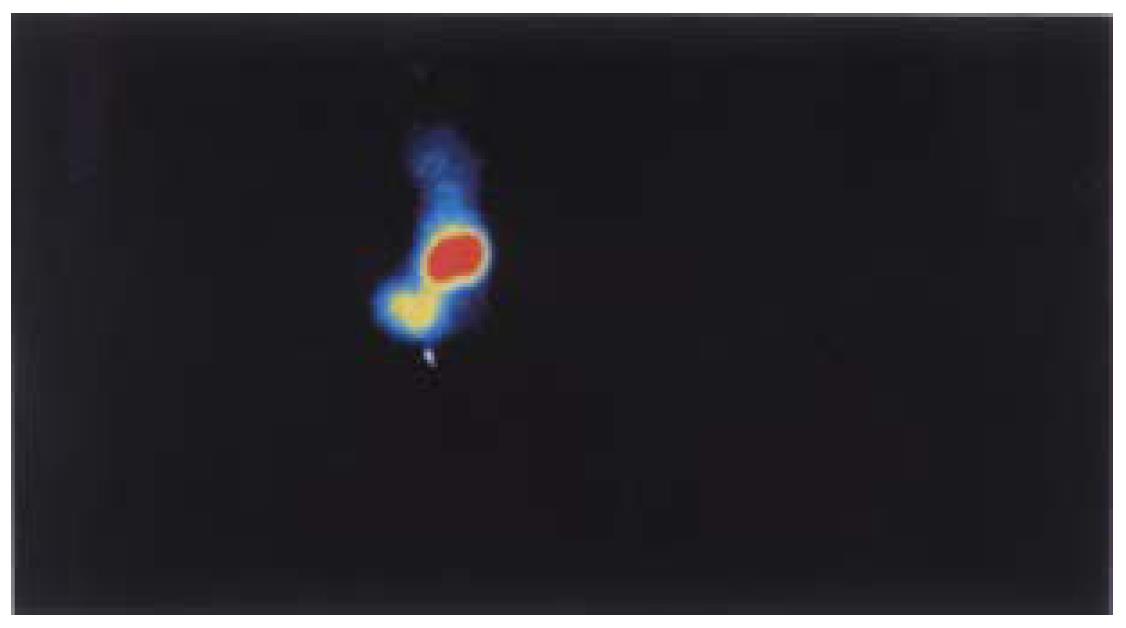
Biodistribution of radioactivity in blood and excised tissues are displayed in Table 2. The preparation localized at the tumors was more than at any organ examined at both 10 h and 24 h after injection, except the kidneys. The lower radioactivity in blood at 24 h suggested fast blood clearance. The imaging results in Figure 6 showed significant tumor uptake at 24 h post-injection.
| Organ | Time after injection (h) | ||
| 4 | 10 | 24 | |
| Blood | 2.21 ± 0.24 | 1.45 ± 0.15 | 0.56 ± 0.09 |
| Kidney | 72.38 ± 4.37 | 70.47 ± 15.23 | 56.45 ± 11.36 |
| Liver | 1.82 ± 0.48 | 1.59 ± 0.31 | 1.43 ± 0.27 |
| Lung | 1.62 ± 0.34 | 1.40 ± 0.17 | 0.75 ± 0.21 |
| Stomach | 1.37 ± 0.39 | 1.05 ± 0.28 | 0.50 ± 0.29 |
| Spleen | 2.35 ± 0.81 | 2.11 ± 0.75 | 1.82 ± 0.85 |
| Large intestine | 1.16 ± 0.34 | 1.42 ± 0.39 | 0.94 ± 0.32 |
| Small intestine | 0.97 ± 0.31 | 0.95 ± 0.18 | 0.62 ± 0.24 |
| Heart | 2.04 ± 0.55 | 1.83 ± 0.48 | 1.17 ± 0.42 |
| Muscle | 1.15 ± 0.20 | 0.77 ± 0.28 | 0.51 ± 0.25 |
| Tumor | 5.14 ± 2.26 | 5.84 ± 2.98 | 6.57 ± 3.01 |
Great efforts have been made to develop a method that can be used for the direct labeling of mAbs with 99mTc[16]. Earlier studies involved the incubation of mAbs with stannous phthalate tartrate solution for up to 21 h at room temperature, which was named "pretinning" method. Clinical success with this method has been claimed by the author[23].
One aim of our study was to further evaluate the role of stannous as a reducing agent in the direct labeling of mAb F(ab') 2 with 99mTc. The difference between the "pretinning" method and this method is that we use GH instead of phthalate-tartrate as transfer ligand and stabilizer to avoid Sn or Tc-colloid formation. To do this, we investigated the effect of the quantity of Sn/GH on the labeling time and efficiency. When the molar ratio of Sn/GH to mAb F(ab')2 was constant, we found that there was no obvious difference on the number of-SH between the reduction time of 20 min and 30 min or even longer[24]. The whole labeling process can be accomplished within 1.5 h. Hnatowich et al[12] reported that labeling efficiency in the case of the stannous ion-reduced a ntibodies was generally in excess of 70%, however, in our method molar ratio of Sn/GH to mAb was an important parameter to obtain good labeling results, and molar ratio of 40:1 or higher were needed to get labeling efficiency of more than 80% (Table 1). The low percentage of free 99mTcO4 and radiocolloid in each sample implied that pH5.3 and GH are the optimal pH value and transfer ligand. Under this condition, the labeled mAb HAb18 F(ab')2 keeps its immunoreactivity. Autoradiography of SDS-PAGE had only one migration of component identical to that of native HAb18 F(ab')2 determination by staining with Coomassie brilliant blue R250 (Figure 2), which demonstrated that S n/GH reduction is mild and does not destroy interchain bridges in mAbs. Labeling efficiency of 2% in control experiments using unreduced HAb18 F(ab')2 indicated that there was no exchange with the low affinity sites and also demonstrated that reduction of disulfides is a necessary initial step in 99mTc direct labeling of antibodies. The bond between SH and Tc is stronger than that of N-Tc or O-Tc which was verified by the challenge assay of 99mTc-HAb18 F(ab')2 in the presence of EDTA. We found that EDTA even at a molar ratio of 10000:1 failed to remove a significant amount of 99mTc, this is in agreement with the results of Rhodes et al[8]. But L-cys at 625:1 remove one-tenth of the label (Figure 5). Despite such instability of the label, there was no in vivo evidence of release of pertechnetate due to no thyroid imaging observed in the whole imaging process (Figure 7). Tumor localization of 99mTc-HAb18 F(ab')2 was successfully demonstrated in a human tumor/nude mouse xenograft model. Biodistribution and imaging results showed the highest tumor uptake at 24 h post-injection. Where as kidney levels were found to be higher in the whole process. Accumulation of radioactivity in the kidney may be the result of retention of this metallic radionuclide by the kidney pro ximal tubule[25], the possible release of 99mTc-labeled cysteine and gluta thione[26]stemming from the radioimmunoconjugate catabolism, and the r elative amount of 99mTc-GH. A technique has been used in patients to block renal tubule uptake of 99mTc-anti-CEA Fab' fragments by amino acid infusion[27].
In conclusion, a radioimmunoimaging conjugate for hepatoma detection was prepared by direct labeling mAb HAb18 F(ab')2-with 99mTc using stannous/g lucoheptonate as reducing agent. Although the labeling efficiency is not satisfactory to some degree, it has several advantapes: simple, easy and quick, besides, the labeled mAb fragment retains its immunoreactivity. Biodistribution and imaging studies reveal that this conjugate is useful for the detection of hepato ma.
The authors are grateful to Dr. Wang Jing for mice imaging and the Department of Nuclear Medicine of Shaanxi People's Hospital for their support of this project.
Edited by Zhu LH
proofread by Sun SM
| 1. | Rhodes BA. Direct labeling of proteins with 99mTc. Int J Rad Appl Instrum B. 1991;18:667-676. [PubMed] |
| 2. | Childs RL, Hnatowich DJ. Optimum conditions for labeling of DTPA-coupled antibodies with technetium-99m. J Nucl Med. 1985;26:293-299. [PubMed] |
| 3. | Ultee ME, Bridger GJ, Abrams MJ, Longley CB, Burton CA, Larsen SK, Henson GW, Padmanabhan S, Gaul FE, Schwartz DA. Tumor imaging with technetium-99m-labeled hydrazinonicotinamide-Fab' conjugates. J Nucl Med. 1997;38:133-138. [PubMed] |
| 4. | Joiris E, Bastin B, Thornback JR. A new method for labelling of monoclonal antibodies and their fragments with technetium-99m. Int J Rad Appl Instrum B. 1991;18:353-356. [PubMed] |
| 5. | Eckelman WC, Meinken G, Richards P. 99m Tc-human serum albumin. J Nucl Med. 1971;12:707-710. [PubMed] |
| 6. | John E, Thakur ML, Wilder S, Alauddin MM, Epstein AL. Technetium-99m-labeled monoclonal antibodies: influence of technetium-99m binding sites. J Nucl Med. 1994;35:876-881. [PubMed] |
| 7. | Mather SJ, Ellison D. Reduction-mediated technetium-99m labeling of monoclonal antibodies. J Nucl Med. 1990;31:692-697. [PubMed] |
| 8. | Rhodes BA, Zamora PO, Newell KD, Valdez EF. Technetium-99m labeling of murine monoclonal antibody fragments. J Nucl Med. 1986;27:685-693. [PubMed] |
| 9. | Pauwels EK, Welling MM, Feitsma RI, Atsma DE, Nieuwenhuizen W. The labeling of proteins and LDL with 99mTc: a new direct method employing KBH4 and stannous chloride. Nucl Med Biol. 1993;20:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Schwarz SW, Connett JM, Anderson CJ, Rocque PA, Philpott GW, Guo LW, Welch MJ. Evaluation of a direct method for technetium labeling intact and F(ab')2 1A3, an anticolorectal monoclonal antibody. Nucl Med Biol. 1994;21:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Qi P, Muddukrishna SN, Torok-Both R, Rahn J, Chen A. Direct 99mTc-labeling of antibodies by sodium dithionite reduction, and role of ascorbate as a stabilizer in cysteine challenge. Nucl Med Biol. 1996;23:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Hnatowich DJ, Virzi F, Fogarasi M, Rusckowski M, Winnard P. Can a cysteine challenge assay predict the in vivo behavior of 99mTc-labeled antibodies? Nucl Med Biol. 1994;21:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sykes TR, Woo TK, Baum RP, Qi P, Noujaim AA. Direct labeling of monoclonal antibodies with technetium-99m by photoactivation. J Nucl Med. 1995;36:1913-1922. [PubMed] |
| 14. | Nakayama M, Wada M, Araki N, Ginoza Y, Terahara T, Harada K, Sugii A, Tomiguchi S, Kojima A, Hara M. Direct 99mTc labeling of human immunoglobulin with an insoluble macromolecular Sn(II) complex. Nucl Med Biol. 1995;22:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Rosenzweig HS, Ranadive GN, Seskey T, Epperly MW, Bloomer WD. A novel method for the non-chromatographic purification of technetium-99m-labeled monoclonal antibodies: a study with B72.3 monoclonal antibody. Nucl Med Biol. 1994;21:171-178. [PubMed] |
| 16. | Mather SJ. Comment on "An improved method of direct labeling monoclonal antibodies with 99mTc" by M. M. Alauddin, L. A. Khawli and A. L. Epstein, Nucl. Med. Biol. 19, 445-454. Nucl Med Biol. 1993;20:375-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Gooden CS, Snook DE, Maraveyas A, Rowlinson-Busza G, Peters AM, Epenetos AA. Direct technetium-99m labeling of three anticancer monoclonal antibodies: stability, pharmacokinetics and imaging. J Nucl Med. 1995;36:842-849. [PubMed] |
| 18. | Chen ZN, Liu YF, Yang JZ. Anti human hepatocellular carcinoma monoclonal antibody and immunohistochemical location of associated antigen P60. Dankelong Kangti Tongxun. 1989;2:33-36. |
| 19. | Qiu K, Chen ZN, Liu ZG, Wang Q, He FC, Qu P, Mi L, Sui YF, Liu YF. Preparation of anti hepatoma McAb HAb18 F(ab')2 and Fab fragments by papainic digestions in different conditions. Disi Junyi Daxue Xuebao. 1995;16:414-417. |
| 20. | ELLMAN GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18594] [Cited by in RCA: 19225] [Article Influence: 769.0] [Reference Citation Analysis (0)] |
| 21. | Thrall JH, Freitas JE, Swanson D, Rogers WL, Clare JM, Brown ML, Pitt B. Clinical comparison of cardiac blood pool visualization with technetium-99m red blood cells labeled in vivo and with technetium-99m human serum albumin. J Nucl Med. 1978;19:796-803. [PubMed] |
| 22. | Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 699] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 23. | Morrison RT, Lyster DM, Alcorn L, Rhodes BA, Breslow K, Burchiel SW. Radioimmunoimaging with 99mTc monoclonal antibodies: clinical studies. Int J Nucl Med Biol. 1984;11:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Bian HJ, Chen ZN, Deng JL, Duan XD, Mi L, Yu XL, Xu LQ. Direct labeling of anti hepatoma monoclonal antibody fragment with 99mTc by an improved pretinning method. Tongweisu. 1999;12:90-94. |
| 25. | Granowska M, Mather SJ, Britton KE, Bentley S, Richman P, Phillips RK, Northover JM. 99mTc radioimmunoscintigraphy of colorectal cancer. Br J Cancer Suppl. 1990;10:30-33. [PubMed] |
| 26. | Hnatowich DJ, Mardirossian G, Rusckowski M, Fogarasi M, Virzi F, Winnard P. Directly and indirectly technetium-99m-labeled antibodies--a comparison of in vitro and animal in vivo properties. J Nucl Med. 1993;34:109-119. [PubMed] |
| 27. | Behr TM, Becker WS, Sharkey RM, Juweid ME, Dunn RM, Bair HJ, Wolf FG, Goldenberg DM. Reduction of renal uptake of monoclonal antibody fragments by amino acid infusion. J Nucl Med. 1996;37:829-833. [PubMed] |