Published online Feb 15, 2000. doi: 10.3748/wjg.v6.i1.74
Revised: September 2, 1999
Accepted: September 18, 1999
Published online: February 15, 2000
AIM: To study the influence of inducers BNF and PB on the stere oselective metabolism of propranolol in rat hepatic microsomes.
METHODS: Phase I metabolism of propranolol was studied by using the microsomes induced by BNF and PB and the non-induced microsome as the control. The enzymatic kinetic parameters of propranolol enantiomers were calculated by regression analysis of Lineweaver-Burk plots. Propranolol concentrations we re assayed by HPLC.
RESULTS: A RP-HPLC method was developed to determine propranol ol concentration in rat hepatic microsomes. The linearity equations for R (+)pr opranolol and S (-)propranolol were A = 705.7C + 311.2C (R = 0.9987) and A = 697.2C+311.4C (R = 0.9970) respectively. Recoveries of each enant iomer were 98.9%, 99.5%, 101.0% at 60 μmol/L, 120 μmol/L, 240 μmol/L respectively. At the concentration level of 120 μmol/L, propranolol enantiomers were metabolized at different rates in different microsomes. The concentration ratio R (+)/S (-) of control and PB induced microsomes increased with time, whereas that of microsome induced by BNF decreased. The assayed enzyme parameters were: 1. Km. Control group: R (+)30 ± 8, S (-) 18 ± 5; BNF group: R (+) 34 ± 3, S (-)39 ± 7; PB group: R (+)38 ± 17, S (-) 36 ± 10. 2. Vmax. Control group: R (+)1.5 ± 0.2, S (-)2.9 ± 0.3; BNF group: R (+)3.8 ± 0.3, S (-)3. 3 ± 0.5 ; PB group: R (+)0.07 ± 0.03, S (-)1.94 ± 0. 07. 3. Clint. Control group: R (+)60 ± 3, S (-) 170 ± 30; BNF group: R (+)111.0 ± 1, S (-) 84 ± 5; PB group: R (+)2.0 ± 2, S (-)56.0 ± 1. The enzyme parameters compared with unpaired t tests showed that no stereoselectivity was observed in enzymatic affinity of three microsomes to enantiomers and their catalytic abilities were quite different and had stereoselectivities. Compared with the control, microsome induced by BNF enhanced enzyme activity to propranolol R (+)enantiomer, and microsome induced by PB showed less enzyme activity to propranolol S (-)-enan tiomer which remains the same stereoselectivities as that of the control.
CONCLUSION: Enzyme activity centers of the microsome were changed in composition and regioselectivity after the induction of BNF and PB, and the stereoselectivities of propranolol cytochrome P450 metabolism in rat hepatic microsomes were likely due to the stereoselectivities of the catalyzing function in enzyme. CYP-1A subfamily induced by BNF exhibited pronounced contribution to propranolol metabolism with stereoselectivity to R (+)-enantiomer. CYP-2B subfamily induced by PB exhibited moderate contribution to propranolol metabolism, but still had the stereoselectivity of S (-)-enantiomer.
- Citation: Li X, Zeng S. Stereoselective propranolol metabolism in two drug induced rat hepatic microsomes. World J Gastroenterol 2000; 6(1): 74-78
- URL: https://www.wjgnet.com/1007-9327/full/v6/i1/74.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i1.74
Propranolol is a nonselective β-adrenergic blocking agent and widely used in clinic as a racemic mixture of R (+) and S (-) enantiomers. It is extensively metabolized and only a small amount of the drug is excreted unchanged[1,2]. As a beta blocking agent, the optical isomers of propranolol exert different beta receptor blocking and membrane stabilizing effects[3], therefore its stereoselective metabolism is of clinical importance. Propranolol is metabolized into a number of products in vivo. These products arise from naphthalene-ring hydroxylation[1], N-dealkylation of the isopropanolamine side-chain and side-chain o-glucuronidation[4,5]. When the influence by the hepatic blood flow[6] and oxygen delivery[7] in vivo is not considered, the metabolism by monooxygenation is mainly responsible for propranolol elimination in hepatic microsomes and O-glucuronidation was shown to be a minor pathway in vivo[2] and in vitro[5].
The oxidative metabolism of propranolol is catalyzed by cytochrome p-450. Exper iments by Otton SV et al[8] and Ishida R et al[9] indicated that multiple isozymes were involved in popanolol metabolism in rat liver microsomes. Nelson et al[10] have observed that stereoselectivity of pro pranolol metabolism in 9000 g liver supernatant differs depending on the positions of metabolism. Although the metabolic fate of propranolol in rat has been studied extensively, the impact of PB and BNF induction on stereoselective propranolol metabolism in rat hepatic microsome was rarely reported. This experiment studied the stereoselective metabolism of propranolol in rat hepatic micro somes induced by BNF and PB and the enzymatic parameters were compared with that of the control.
R (+) and S (-)-propranolol (hydrochlor ide), β-naphthoflavone (BNF), phenobarbital (PB) NADP and NADPH were supplied by Sigma Chemical Co. (St. Louis, MO, USA). Tris-hydroxymethyl aminomethane (Gibco BRL) and bovine serum albumin (Serva) were purchased from Shanghai Reagent Station. All other chemicals were obtained from the common commercial sources.
Tris-HCl buffer ( 0.1 mol/L, pH 7.4 ): 1.21 g of Tris-hydroxymeth yl aminomethane was dissolved in 60 mL of water. The solution was adjusted to pH 7.4 by concentrated hydrochloride acid and then diluted with water to the desired volume of 100 mL. This solution was used to prepare rat hepatic microsome.
Ammonium acetate buffer: 4.0 g of ammonium acetate was dissolved in 10 mL glacial acetic acid and then diluted with water to the desired volume of 1000 mL ( pH 4.0 ). This solution was used to prepare mobile phase.
Sprague-Dawley rats ( male, 160 g-200 g ) were divided into three groups. One group received i.p. injection of sodium PB dissolved in physiological saline (0.9% NaCl) (80 mg/kg·d) for 3 days, another group, BNF in cornoil (80 mg/kg·d) for 3 days and the last group received nothing as the non-treated control. About 24 h after the last treatment and with no food supplied for 16 h before taking the livers, the rats were sacrificed by decapitation. Liver samples were excised and perfused by the ice-cold physiological saline to remove blood and homogenized in ice-cold Tris-HCl buffer. Hepatic microsomes were prepared with the ultracentrifugation method described by Gibbson GG et al[11]. All manipulations were carried out in a cold bath. Pellets were re-suspended in sucrose-Tris buffer (pH 7.4) (95:5) and immediately stored at -30 °C.
Protein concentrations of the microsomal preparations were measured by the method of Lowry et al[12] using arystalline bovine serum albumin as the protein standard.
0.5 mL incubation mixture containing 1 mg/mL microsomal protein per milliliter (85 mmol/L Tris-HCl buffer (pH 7.4), 50 mmol/L nico tinamide, 15 mmol/L MgCl2, 3 mg/mL DL-isocitric acid tri-sodium salt, 0.4 units/mL isocitric dehydrogenase) was used Phase I metabolism was performed with 0.5 mL of the mixture bubbled with oxygen for 1 min and R (+) or S (-)propranolol enantiomer as the substrate. After 5min pre-incubation under air at 37 °C, reaction was started by adding 10 µL of NADPH regenerating system (10 mg NADP and 3 mg NADPH in 100 µL of 1% NaHCO3). The reaction was stopped after the indicated time by adding 0.5 mL of methanol and centrifuged at 4000 r/min for 10 min. 10 µL of the supernatant was sampled into HPLC.
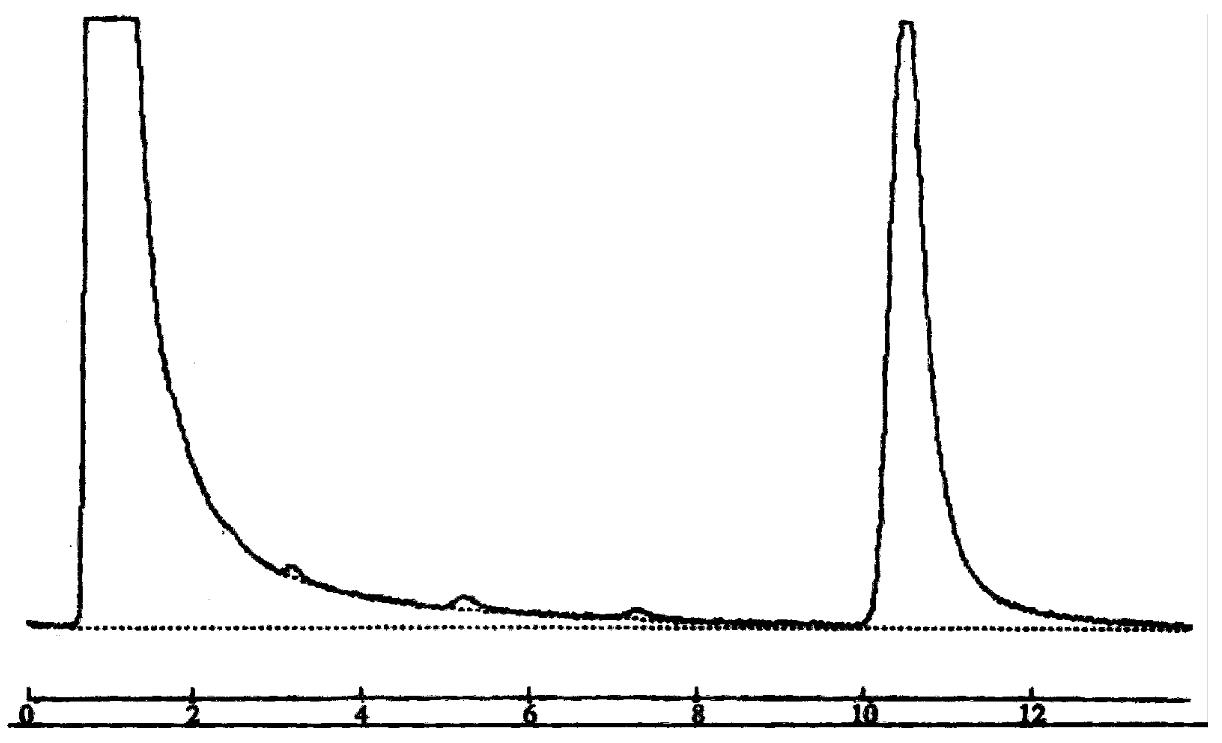
A HPLC procedure was established to assay propranolol enantiomers in rat hepatic microsomes. After the termination of the reaction with methanol, 10 µL of the sample was applied to a reversed phase column (Shim-pack CLC-ODS 15 cm × 0.6 cm id, 10 µm particle size). Propranolol was monitored with a UV detector at 290 nm. The mobile phase was made up with ammonium acetate buffer (pH 4.0)-methanol ( 50:50 ). The flow rate was 1.0 mL/min. Figure 1 shows the typical elution of propranolol in incubation solution.
The maximum velocity (Vmax) and the Michaelis-Menten constant (Km) values for propranolol were determined by regression analysis of Lineweaver-Burk plots. The -x ± s of three determinations of Vmax and Km was calculated for each substrate and metabolic reaction. Intrinsic clearance was calculated by the ratio of Vmax/Km. The statistical difference between propranolol enantiomers was tested using an unpaired t test.
Linearity Drug-free microsomes were spiked with increasing concentrations of propranolol enantiomers ( 10 µmol/L-620 µmol/L ). The solution was constituted according to “Incubation of propranolol with rat hepatic microsomes” with no occurrence of metabolism reaction. Propranolol enantiomers were assayed by HPLC preciously described. Standard calibration curves were constructed by performing a linear regression analysis of the peak area (Y) of propranolol enantiomers versus their concentrations (X), i.e., R (+)propranolol: Y = 705.7 + 311.2X, r = 0.9987; S (-)propranolol: Y = 697.2 + 311.4X, r = 0.9970. The limit of detection (single-to-noise ratio = 3) for propranolol was 3 μmol/L.
Precision and accuracy The spiked drug-free microsomes at 3 concentration levels (60 μmol/L, 120 μmol/L and 240 μmol/L) were assayed following the procedure of 2.1.1. Results were listed in Table 1.
| Target concentrations (μmol/L) | Recovery (%) | Precisions (RSD, %) | |
| Intra-assay (n = 3) | Inter-assay (n = 3) | ||
| 60 | 98.8 | 5.1 | 5.6 |
| 1 2 0 | 99.5 | 3.5 | 4.8 |
| 240 | 101.0 | 3.2 | 5.3 |
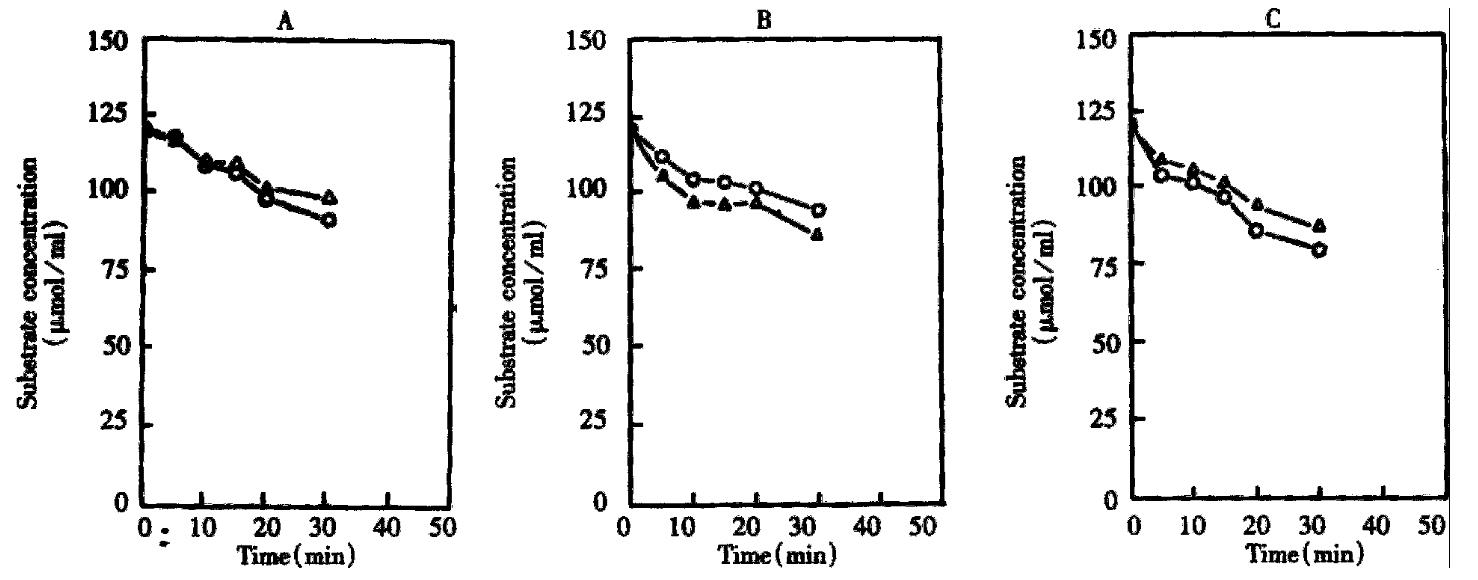
Concentration-time curves and variation of the ratio of R (+)/S (-) propranolol concentration in microsomes after incubation of different time Phase I metabolism was performed with 0.5 mL of the mixture and 60 μmol of propranolol enantiomers as the substrate. The incubation procedure was carried out according to 1.3. and 1 mL of methanol was added to stop the reaction at 0, 40, 80, 160, 320 min respectively. The mixtures were then analyzed by HPLC. Results are shown in Figure 2 and Table 2.
It was indicated that at the propranolol concentration level of 120 μmol/L, propranolol enantiomers were metabolized in different rate in different microsomes. The ratio of R (+)/S (-) propranolol concentration in incubation media in control and PB group increased, whereas that in BNF group dec reased. The ratio of R (+)/S (-) propranolol concentration in BNF group was signi ficantly different with the corresponding ratio in control group or PB group at 15, 20 and 30 min (P < 0.05, 0.01).
Enzymatic kinetic parameters for propranolol metabolism in liver microsomes from control, BNF and PB induced rats The enzymatic kinetic parame ters of propranolol enantiomers were calculated by Lineweaver-Burk method with the substrate concentrations of 20 μmol/L-600 μmol/L in three forms of rat hepatic microsomes after 10 min incubation (1.3).The results were listed in Table 3.
| Group | Enantiomer | Km μmol/L | Vmax mmol/g/min | Clint L/min/g protein | R( + )Vmax: S( - )Vmax |
| Control | R( + ) | 30 ± 8 | 1.5 ± 0.2b | 60 ± 3b | 0.5 |
| S( - ) | 18 ± 5 | 2.9 ± 0.3 | 170 ± 30 | ||
| BNF | R( + ) | 34 ± 3 | 3.8 ± 0.3fh | 111.0 ± 1afh | 1.14 |
| S( - ) | 39 ± 7d | 3.3 ± 0.5g | 84 ± 5eh | ||
| P B | R( + ) | 38 ± 17 | 0.07 ± 0.03ef | 2.0 ± 2cf | 0.038 |
| S( - ) | 36 ± 10d | 1.94 ± 0.07e | 56.0 ± 1e |
Km of propranolol enantiomers in control group had no stereoselectivity (P > 0.05), whereas Vmax and Clint had stereoselectivity of S (-)-propranolol (P < 0.01). For BNF induced microsome, Km and Vmax had no stereoselectivity between R (+), S (-)-propranolol (P > 0.05), and Clint had significant differenc e between the two enantiomers (P < 0.05). For PB group, Km had no stereosele ctivity (P > 0.05), and Vmax, Clint had stereoselectivity of S (-)-proprano lol (P < 0.001).
Comparing the enzymatic parameters of R (+)-propranolol among three microsomes, Km had no statistical difference (P > 0.05), whereas Vmax and Clint had stat istical differences (P < 0.05, 0.01 or 0.001); compared with the control group, Vmax for BNF group increased 2.5 times and that for PB group decreased 20 times; clint for BNF and PB group increased or decreased 1.8 and 30 times, respectively. With the same way to compare those parameters of S (-)-propranolol, Kms for BNF and PB group increased 2.2 and 2.1 times, respectively, but had no statistical difference with each other; Vmax for PB group decreased about 1.5 times and that for BNF group nearly remained the same, in addition, no statisti cal difference was found between PB and BNF group; Clint for BNF and PB group decreased 2 times and 1.5 times respectively and there was significant difference between BNF and PB group.
In this in vitro study, stereoselectivity of propranolol occurred in catalyzing velocity and intrinsic clearance in control group, and no stereoselectivity was observed in enzyme affinity to the substrate. The introduction of BNF and PB caused changes in the composition of CYP subfamilies and therefore influenced the stereoselective catalyzing ability of microsome to propranolol metabolism, or even reversed the sequence of stereoselectivity, whereas the affinity of enzyme to substrate remained nearly the same and had no stereoselectivity. This phenomenon indicated that, regio-structure of binding site in the activity center of enzyme was almost unchanged, and that of the catalyzing site was significantly changed in propranolol metabolism in rat hepatic microsomes after the introduction of PB and BNF, the influence of BNF and PB induction had reversed effect on the catalyzing stereoselectivity of microsome to propranolol.
BNF is an inducer of CYP-1A subfamily[13-15] and PB is that of CYP-3A[15], CYP-2B subfamily[16,17] (IIB1 and IIB2[18]). Different kinds of cytochrome P-450 may be involved in propranolol metabolism, depending on the metabolic positions[10]. CYP-1A is suggested to catalyze 4, 5-hydroxylation and N-desisopropylation stereoselectively[19,20]. CYP-1A2 accounts for about 10% to 15% of the total CYP content of human liver and is the major enzyme involved in the metabolism of propranolol[21]. Another subfamily CYP-2D6 mainly catalyzes 4, 5 and 7-hydro xylation stereoselectively[22,23] and it has been confirmed that CYP-2D6 does not contribute to N-desisopropylation of propranolol[8]. N-desisopropylation in propranolol enantiomer metabolism is mainly mediated by CYP-1A2[24,25]. Masubuchi Y et al[26] reported that there is competition between enantiomers of propranolol for the enzyme, probably the same enzyme, a cytochrome P450 isozyme in the CYP-2D subfamily. All of these showed that different cytochrome subfamilies have different functions in metabolism of propranolol enantiomers and the optical isomers of propranolol have different stereoselectivities in metabolism. Our results indicated that CYP-1A was involved in propranolol metabolism and showed the stereoselectivity of R (+)-enantiomer in general. CYP-3A, CYP-2B subfamily does not play a main role in propranolol metabolism in vitro, though it showed the stereoselectivity of S (-)-enanti omer.
Edited by Wang XL
| 1. | Walle T, Oatis JE, Walle UK, Knapp DR. New ring-hydroxylated metabolites of propranolol: species differences and stereospecific 7-hydroxylation. Drug Metab Dispos. 1982;10:122-127. [PubMed] |
| 2. | Bargar EM, Walle UK, Bai SA, Walle T. Quantitative metabolic fate of propranolol in the dog, rat, and hamster using radiotracer, high performance liquid chromatography, and gas chromatography-mass spectrometry techniques. Drug Metab Dispos. 1983;11:266-272. [PubMed] |
| 3. | Stark G, Stark U, Lueger A, Bertuch H, Pilger E, Pietsch B, Tritthart HA, Lindner W. The effects of the propranolol enantiomers on the intracardiac electrophysiological activities of Langendorff perfused hearts. Basic Res Cardiol. 1989;84:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Masubuchi Y, Yamamoto LA, Uesaka M, Fujita S, Narimatsu S, Suzuki T. Substrate stereoselectivity and enantiomer/enantiomer interaction in propranolol metabolism in rat liver microsomes. Biochem Pharmacol. 1993;46:1759-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Thompson JA, Hull JE, Norris KJ. Glucuronidation of propranolol and 4'-hydroxypropranolol. Substrate specificity and stereoselectivity of rat liver microsomal glucuronyltransferases. Drug Metab Dispos. 1981;9:466-471. [PubMed] |
| 6. | Pirttiaho HI, Sotaniemi EA, Pelkonen RO, Pitkänen U, Anttila M, Sundqvist H. Roles of hepatic blood flow and enzyme activity in the kinetics of propranolol and sotalol. Br J Clin Pharmacol. 1980;9:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Hickey PL, McLean AJ, Angus PW, Choo EF, Morgan DJ. Increased sensitivity of propranolol clearance to reduced oxygen delivery in the isolated perfused cirrhotic rat liver. Gastroenterology. 1996;111:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Otton SV, Gillam EM, Lennard MS, Tucker GT, Woods HF. Propranolol oxidation by human liver microsomes--the use of cumene hydroperoxide to probe isoenzyme specificity and regio- and stereoselectivity. Br J Clin Pharmacol. 1990;30:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ishida R, Obara S, Masubuchi Y, Narimatsu S, Fujita S, Suzuki T. Induction of propranolol metabolism by the azo dye sudan III in rats. Biochem Pharmacol. 1992;43:2489-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Nelson WL, Bartels MJ. Stereoselectivity in the aromatic hydroxylation of propranolol in the rat: use of deuterium labeling and pseudoracemic mixtures. Drug Metab Dispos. 1984;12:382-384. [PubMed] |
| 11. | Gibbson GG, Shett P. Introduction to drug metabolism (Second Edition). London: Blackie Academic & Professional. 1994;217-221. |
| 12. | LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 13. | Bachmann K, Sanyal G, Potter J, Schiavone R, Loch J. In vivo evidence that theophylline is metabolized principally by CYP1A in rats. Pharmacology. 1993;47:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Takahashi N, Harttig U, Williams DE, Bailey GS. The model Ah-receptor agonist beta-naphthoflavone inhibits aflatoxin B1-DNA binding in vivo in rainbow trout at dietary levels that do not induce CYP1A enzymes. Carcinogenesis. 1996;17:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Pichard L, Gillet G, Bonfils C, Domergue J, Thénot JP, Maurel P. Oxidative metabolism of zolpidem by human liver cytochrome P450S. Drug Metab Dispos. 1995;23:1253-1262. [PubMed] |
| 16. | Chang TK, Chen G, Waxman DJ. Modulation of thiotepa antitumor activity in vivo by alteration of liver cytochrome P450-catalyzed drug metabolism. J Pharmacol Exp Ther. 1995;274:270-275. [PubMed] |
| 17. | Nims RW, Lubet RA, Diwan BA, Mellini DW, Utermahlen WE, Thomas PE. Hepatic cytochrome P450 2B induction by ethyl/phenyl-substituted congeners of phenobarbital in the B6C3F1 mouse. J Biochem Toxicol. 1994;9:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Capdevila JH, Karara A, Waxman DJ, Martin MV, Falck JR, Guenguerich FP. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem. 1990;265:10865-10871. [PubMed] |
| 19. | Fujita S, Umeda S, Funae Y, Imaoka S, Abe H, Ishida R, Adachi T, Masuda M, Kazusaka A, Suzuki T. Regio- and stereoselective propranolol metabolism by 15 forms of purified cytochromes P-450 from rat liver. J Pharmacol Exp Ther. 1993;264:226-233. [PubMed] |
| 20. | Ching MS, Bichara N, Blake CL, Ghabrial H, Tukey RH, Smallwood RA. Propranolol 4- and 5-hydroxylation and N-desisopropylation by cloned human cytochrome P4501A1 and P4501A2. Drug Metab Dispos. 1996;24:692-694. [PubMed] |
| 21. | Brøsen K. Drug interactions and the cytochrome P450 system. The role of cytochrome P450 1A2. Clin Pharmacokinet. 1995;29 Suppl 1:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Masubuchi Y, Hosokawa S, Horie T, Suzuki T, Ohmori S, Kitada M, Narimatsu S. Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes. The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos. 1994;22:909-915. [PubMed] |
| 23. | Rowland K, Ellis SW, Lennard MS, Tucker GT. Variable contribution of CYP2D6 to the N-dealkylation of S-(-)-propranolol by human liver microsomes. Br J Clin Pharmacol. 1996;42:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Yoshimoto K, Echizen H, Chiba K, Tani M, Ishizaki T. Identification of human CYP isoforms involved in the metabolism of propranolol enantiomers--N-desisopropylation is mediated mainly by CYP1A2. Br J Clin Pharmacol. 1995;39:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Narimatsu S, Mochida M, Matsumoto T, Masubuchi Y, Horie T, Nagata K, Funae Y, Cho AK, Suzuki T. Cytochrome P450 enzymes involved in the enhancement of propranolol N-desisopropylation after repeated administration of propranolol in rats. Chem Biol Interact. 1996;101:207-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Masubuchi Y, Yamamoto LA, Uesaka M, Fujita S, Narimatsu S, Suzuki T. Substrate stereoselectivity and enantiomer/enantiomer interaction in propranolol metabolism in rat liver microsomes. Biochem Pharmacol. 1993;46:1759-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |