Published online Feb 15, 2000. doi: 10.3748/wjg.v6.i1.57
Revised: September 2, 1999
Accepted: September 20, 1999
Published online: February 15, 2000
AIM: To clone and identify the whole cDNA of MXR7 gene and to find out its expression in human HCC, and normal tissues.
METHODS: The DNA primers were designed and synthesized accordin g to the whole cDNA sequence of MXR7 gene. The cDNA of human HCC was taken a s the template while the cDNA of MXR7 gene was synthesized by polymerase cha in reaction (PCR). Recombinant DNA conforming to reading frame was constructed b y connecting purified PCR product of the cDNA of MXR7 gene with expression v ector pGEX-5X-1 of fusion protein. The plasmid MXR7/pGEX-5X-1 was identi fied by sequencing. Using 32P labeled MXR7 cDNA as probe, MXR7 mRNA expression was detected by Northern blot analysis in 12 different human no rmal tissues, 7 preoperatively untreated non-liver tumor tissues, 30 preoperati vely untreated HCC, the paracancerous liver tissues and 12 normal liver tissues samples.
RESULTS: Restriction enzyme and sequence analysis confirmed tha t the insertion sequence in vector pGEX-5X-1 was the same as the cDNA sequence of MXR7 gene. Northern blot analysis showed no expression of MXR7 mRNA in 12 kinds of normal human tissues including liver, 7 tumor tissues in other si tes and 12 normal liver tissues, the frequencies of MXR7 mRNA expression in HCC and paracancerous liver tissues were 76.6% and 13.3%, respectively. The frequency of MXR7 mRNA expression in HCC without elevation of serum AFP and in HCC < 5 cm was 90% (9/10) and 83.3% (5/6), respectively.
CONCLUSION: MXR7 mRNA is highly expressed in human HCC, which is specific and occurs at an early stage of HCC, suggesting MXR7 mRNA can be a tumor biomarker for HCC. The detection of MXR7 mRNA expression in the biopsied liver tissue is helpful in discovering early subclinical liver cancer in those with negative serum AFP.
- Citation: Zhou XP, Wang HY, Yang GS, Chen ZJ, Li BA, Wu MC. Cloning and expression of MXR7 gene in human HCC tissue. World J Gastroenterol 2000; 6(1): 57-60
- URL: https://www.wjgnet.com/1007-9327/full/v6/i1/57.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i1.57
The cloned mitoxantrone-resistant 7 (MXR7) gene contains the whole open reading frame (ORF) screened by differential hybridization from λ complementary DNA expression library, from the mitoxantrone-resistant human gastric carcinoma cell line EPG85-257RNOV and is a full-length cDNA of 2263 bp encoding a putative protein of 580 amino acids[1]. With MXR7 mRNA expression at high level in hepatocellular carcinoma (HCC), its temporospatial expression in human resembles α-fetoprotein ( AFP ) gene expression[2]. To probe into and clarify the relation between MXR7 gene and the tumorigenesis and progression and clinical diagnosis of HCC, we analyzed 30 human HCC and the corresponding parac ancerous liver tissues, 12 normal liver tissues, 12 different normal tissues and 7 non-liver tumor tissues.
Tissue specimens used in the present study were sampled from 30 preoperatively untreated patients with pathologically confirmed HCC and the non-tumorous liver tissue ( 2 cm away from the carcinoma), including 28 males and 2 females, aged 21-70 years with a mean of 50.1 years. The controls were normal liver t issues from 12 patients with hepatic hemangioma, 12 different normal tissues from 2 accidental deaths, and 7 preoperatively untreated non-liver tumor tissues. The surgical specimens were immediately cut into small pieces under aseptic condition, snap frozen and stored in liquid nitrogen until use.
Expressing vector pGEX-5X-1 (4.9 kb) of fusion protein was provided by Max-Planck Institute.
E. coli DH5α was preserved in our laboratory.
The sequences of the sense and antisense primers of MXR7 were 5’GCGAATTCTCCCTGCGAAGCAGGATG3’ with the EcoRI (GAATTC) restriction site and 5’CGCTCGAGTCAGTGCACCAGGAAGAA3’ with the restriction site XhoI (CTCGAG), respectively. The amplifying DNA fragments of above primers were 1774 bp and all primers were synthesized by Shanghai Biochemistry Institute, Chinese Academy of Sciences.
The 1774 bp fragments of MXR7 gene were amplified from the cDNA of human HCC by PCR and cloned into the expressing vector pGEX-5X-1. The positive clones selected from the transfected DH5α were performed as described in the reference[3]. The constructed plasmids (designated as MXR7-/pGEX-5X-1) were identified by the restriction enzyme analysis and verified by sequencing.
The purified PCR product was labeled with 32P-dCTP as the probe by random primer method as described in the reference[4] with Prime-a-Gene Labeling System (Promega) and purified with QIAquick Nucleotide Removal Kit Protocol (Qiagen).
RNA extraction Total RNA was extracted using TRIZOL reagent (Gibco, BRL). The RNA from about 1.0 g tissue mass was dissolved in 0.5 mL water pretreated with diethylpyrocarbonate (DEPC) and then stored at -85 °C for use.
Preparation of the hybridization membrane Each 50ìg denatured total RNA was transferred to nitrocellulose filters (BA85, Schleicher Schuell) by electrophoresis performed on 1% agarose-formaldehyde gels. The filters were dried in a vacuum drying oven at 80 °C for 2 h and then sealed in a plastic bag for use.
Northern blot analysis Northern bloting was performed as described in the reference[5]. The amount and quality of the loaded RNA samples were evaluated carefully by ethidium bromide-stained 28S and 18S rRNAs, RNA samples with evidence of degradation and blots that fail to hybridize normally expressed genes were discarded.
Percentage of the specimens was compared by χ2 test.
Through phone calls or by mails and re-examination at the outpatient department, we had followed 22 of 30 patients for more than 2 years or until death for further analysis.
The expected size of the amplified MXR7 fragment was about 1800 bp. The expected size of the recombinant plasmid MXR7/pGEX-5X-1 after enzyme-cut by EcoR-I and Xho-I was about 4900 bp and 1800 bp, respectively. Sequence analysis of MXR7/pGEX-5X-1 confirmed that the insertion sequence of vector pGEX-5X-1 was the same as the translational region sequence of MXR7 cDNA.
The results of Northern blot analysis were classified into positive and negative.
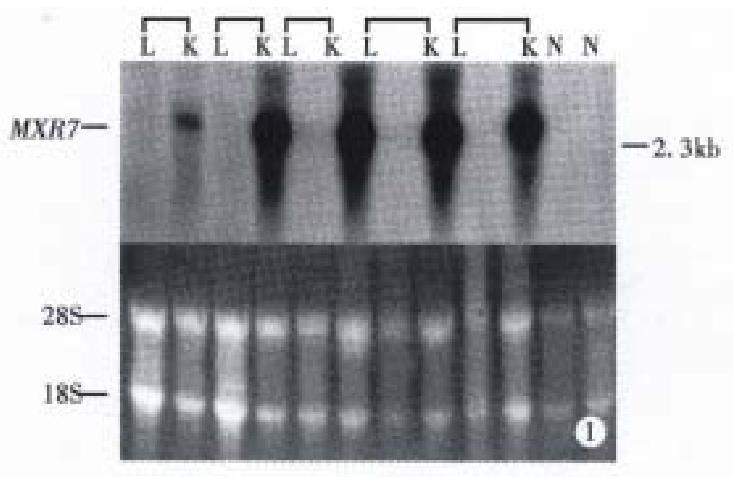
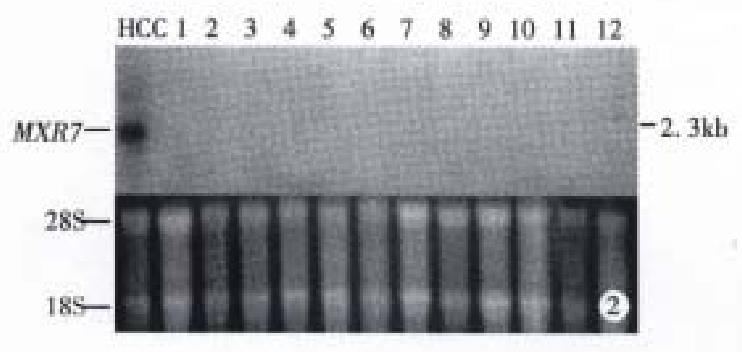
Expression of MXR7 mRNA in HCC, normal tissue and tumor of other ana tomical sites Northern blot analysis showed: ① No expression of MXR7 mRNA on 12 normal liver tissues of patients with hepatic hemangioma, MXR7 mRNA was detected at low level in only 4 (13.3%) of 30 corresponding paracancerous tissue (all were cirrhosis), all of which had intrahepatic portal vein tumor thrombus and multiple daughter modules. By comparison, the frequency of expres sion of 2.3 kb MXR7 mRNA at high level was 76.7% (23 of 30 cases) in HCC samples, significantly higher than that with elevated serum AFP ≥ 400 μg/L (43.3%, 13 of 30 cases, P < 0.01) in this group (Figure 1, Table 1 and Table 2). ② MXR7 mRNA was undetectable in 12 different normal tissues, including liver, lung, kidney, heart, brain, small intestine, colon, testis, spleen, gastric, cyst and pancreas (Figure 2). ③ Among tumors of other anatomical sites including 3 gastric adenocarcinomas, 1 sigmoid adenocarcinoma, 1 malignant mesothelioma, 1 uterine myo-adenoma and 1 familial colonic adenomatosis, MXR7 mRNA was undetectable too (Figure 3).
| Clinical feature | n | MXR7 mRNA overexpression (%) |
| Serum AFP (μg/L) | ||
| ≥ 400 | 13 | 10 (76.9) |
| 30-400 | 7 | 4 (57.1) |
| ≤ 30 | 10 | 9 (90.0) |
| Tumor size (cm) | ||
| ≥ 5 | 24 | 18 (75.0) |
| < 5 | 6 | 5 (83.3) |
| Portal vein tumor thrombus | ||
| Yes | 24 | 17 (70.8) |
| No | 6 | 6 (100.0) |
| Daughter tumor | ||
| Yes | 17 | 12 (70.6) |
| No | 13 | 11 (84.6) |
| Serum HBsAg | ||
| Yes | 24 | 19 (79.2) |
| No | 6 | 4 (66.7) |
| Age (years) | ||
| ≥ 50 | 16 | 12 (75.0) |
| < 50 | 14 | 11 (78.6) |
Clinicopathological profiles of 30 studied cases with HCC These included 28 men and 2 women, aged 21-70 years with a mean of 50.1 years. Serum HBsAg was positive in 24 cases (80%). The serum AFP level was above 400 μg/L in 13 (43.3%) and below 30 μg/L in 10 (33.3%). The tumor size was < 5 cm (small HCC) in 6 and large in 24 (large HCC). Histologically, all 30 tumors had invasion (invasive HCC), with portal vein thrombus in 24 (80%) and distant satellite nodule in 17 (56.7%). The differentiation of all tumors were Edmondson III-IV grade.
MMXR7 mRNA expression in relation to clinico-pathological features As shown in Table 2, MXR7 mRNA expression did not correlate with s erum AFP elevation, tumor size, portal vein tumor thrombus, daughter nodules, HB sAg seropositivity and age. The frequency of MXR7 mRNA expression in HCC was 70% (14 of 20 cases) with elevated serum AFP > 30 μg/L, but 90% ( 9 of 10 cases ) with serum AFP ≤ 30 μg/L. In HCC < 5 cm, the frequency (83.3%, 5 of 6 cases) of MXR7 mRNA expression was higher than that with elevated serum AFP (33.3%, 2/6 cases).
Association between MXR7 mRNA expression in surgical specimens of HCC and prognosis of the patients In our study, 12 out of 22 patients survived for 2 years postoperatively, 10 of whom had no signs of recurrence nor metastasis, in the other two, there had been one recurrent tumor in the rig ht and left lobes, respectively. The average survival period of the 10 deceased of 22 patients was 8.0 months (2-25 months). The main causes of death were tumor recurrence, portal vein tumor thrombosis and ascites. The survival rates for 1 year and 2 years were 59.1% and 54.5%, respectively. Our data did not show that MXR7 mRNA expression was correlated with the prognosis of patients.
Although an elevated serum AFP level is regarded as a tumor marker for HCC, the frequency of elevated serum AFP in HCC is about 60% up to date, which is much lower in small HCC. The study showed no detectable expression of MXR7 mRNA in 12 different normal tissues including liver, 7 non-liver tumor tissues and 12 normal liver tissues and the frequencies of MXR7 mRNA expression in HCC and t he corresponding paracancerous cirrhotic tissues were 76.7% and 13.3%, respect ively. These findings indicate that MXR7 mRNA overexpression in HCC is common and specific, suggesting that MXR7 gene served as a sensitive marker for HCC.
Our observations confirmed only 33.3% (2 of 6 cases) of the patients with small HCC( < 5 cm) had an elevated serum AFP level ( > 30 μg/L), which was lower than that of MXR7 mRNA overexpression (83.3%, 5 of 6 cases), and the frequency of MXR7 mRNA overexpression in HCC was 70% (14 of 20 cases) with serum AFP elevation but 90% (9 of 10 cases) without serum AFP elevation, suggesting that MXR7 gene may be a sensitive early tumor marker for HCC and the detection of MXR7 mRNA expression in liver biopsied tissues was able to discover small HCCs in the subclinical stage with negative serum AFP.
Four cases of MXR7 mRNA expression in paracancerous cirrhotic tissues were 1 with two 4cm tumor masses in left-external and right-posterior hepatic lobe and 3 cases with >10 cm tumor masses accompanied by multiple daughter tumors, from which we can presume that retained carcinoma cells in the paracancerous h epatic tissue of the surgical specimens with large tumor mass and tumor invasion may be the cause of MXR7 mRNA expression in the paracancerous liver tissue, this suggests that detection of MXR7 mRNA expression in the paracancerous liver tissue can serve as one of indicators whether the tumor in completely resec ted or not and also as referential value in deciding further treatment.
The cDNA sequence of MXR7 is 100% homologous to the cDNA of Glypican 3 (GPC3)[1], which is a developmentally regulated gene localized to chromosome Xq26[6], GPC3 sequences are very well conserved through evolution, being highly homologous among mice, rats and human being[7]. GPC3 is believed to be involved in morphogenesis and growth control during development regula ted by cell morphology and cell density at the transcription level[8].
The familial aggregation and the heredity of susceptibility of the patients with HCC is well documented and the frequency of HCC among males is as about 10 folds that of females. The epidemiology study showed that the effect of heredity on maternal side is much higher than that of paternal one. Clinically, HCC responds poorly to the chemotherapy, which might be correlated with the common overexpre ssion of MXR7 mRNA in human HCC.
MXR7 mRNA expression is closely related to oncogenesis and progression, the heredity of susceptibility and the poor therapeutic effect of chemotherapy of HCC, but the molecular mechanism remains unclear. The construction of recombinant plasmid MXR7/pGEX-5X-1 expressing fusion portion is useful for studying the correlation of MXR7 and HCC with the structure and function of the gene product.
Edited by Wu XN and Ma JY
Proofread by Miao QH
| 1. | Lage H, Dietel M. Cloning and characterization of human cDNAs encoding a protein with high homology to rat intestinal development protein OCI-5. Gene. 1997;188:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57:5179-5184. [PubMed] |
| 3. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A labora-tory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press. 1989;55-60. |
| 4. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A labora-tory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press. 1989;502-506. |
| 5. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A labora-tory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press. 1989;363-371. |
| 6. | Veugelers M, Vermeesch J, Reekmans G, Steinfeld R, Marynen P, David G. Characterization of glypican-5 and chromosomal localization of human GPC5, a new member of the glypican gene family. Genomics. 1997;40:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Li M, Pullano R, Yang HL, Lee HK, Miyamoto NG, Filmus J, Buick RN. Transcriptional regulation of OCI-5/Glypican 3: elongation control of confluence-dependent induction. Oncogene. 1997;15:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Li M, Choo B, Wong ZM, Filmus J, Buick RN. Expression of OCI-5/glypican 3 during intestinal morphogenesis: regulation by cell shape in intestinal epithelial cells. Exp Cell Res. 1997;235:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |