Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.550
Revised: August 20, 1999
Accepted: September 5, 1999
Published online: December 15, 1999
- Citation: Chen K, Han BG, Ma XK, Zhang HQ, Meng L, Wang GH, Xia F, Song XG, Ling SG. Establishment and preliminery use of hepatitis B virus preS1/2 antigen assay. World J Gastroenterol 1999; 5(6): 550-552
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/550.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.550
Hepatitis B virus (HBV) is a DNA virus with an envelope, its membrane protein gene preS/S have three parts-preS1, preS2 and S, there is a translation initiation codon ATG in each of them. By different ATG, the membrane protein gene can be translated into three kinds of products, i.e. the large protein ( preS1 + preS2 + S ), medium ( preS2 + S) and small (S) (also called primary protein). In patients with acute hepatitis B, PreS antigen occurred together with other HBV markers and parallels the level of HBV DNA. Disappearance of PreS antigen predicts a benig n outcome[1]. Its presence in chronic hepatitis B is somewhat correlated with HBsAg, the ratio of PreS1/HBsAg antigen titers can reflect the level of DNA replication[2]. Meanwhile, the PreS domain in the membrane prote in include the binding sites for hepatocyte receptor and a number of epitopes of B cells and T cells. The PreS domain possesses very strong immunogenicity in mouse and man. In mice with consanguinity, the PreS domain can induce broad-spectrum protective antibodies and overcome their nonresponsiveness to protein S. In human body, it has been proved the PreS domain is an effective immunogen at both B and T cell levels. The antibodies directing against synthetic peptides (PreS: 21-47 ) mimicking receptor-binding sites can protect chimpanzee from HBV infection[3]. During the disease course appearance of antibody against PreS1 is considered an early marker for elimination of HBV infection[4,5]. Therefore PreS antigen/ antibody is a pair of important indexes for diagnosis of hepatitis B. As one part of a research project granted by the EC Foundation, we have prepared a recombinant PreS protein with high purity, a polyclonal anti -PreS antibody and established the ELISA method for detection of PreS antigen, preliminary clinical studies had been carried out.
HRP-conjugated murine anti-human IgG was provided by Department of Molecular Immunology, Institute of Basic Medical Sciences, Academy of Military Medical Sciences: HRP-conjugated murine anti-human HBs were provided by the Department of Immunology, No.302 Hospital of PLA; and HRP-conjugated sheep anti-rabbit IgG was provided by the Department of Molecular Biology, No.307 Hospital of PLA.
They were provided by the Central Blood Bank of PLA units under Beijing Command and No.301, 302 Hospitals of PLA.
The engineered E. coli for hepatitis B viral PreS/2 recombinant antigen was provided by Department of Virology, Max-Plank Institute of Biochemistry, Germany. The PreS1/2 recombinant antigen containing PreS1:10-108 PreS2: 1-55/S1-22 (ayw subtype) was expressed by using pQe8 (Quiagen) expression vector and six histidin residuals were fused to the N-terminal of the product, which was purified by affinity chromatography using Ni-NTA agarose column (Quiagen) to a purity above 95%. The purified protein was stored at -20 °C for use.
New Zealand large-ear white rabbits were immunized by four injections of renatured PreS recombinant antigen, once each on the wk 0, wk 2, wk 7 and wk 11. One hundred micrograms of the antigen were injected subcutaneousl y on multiple sites at one time. In the first injection, Freund’s complete adju vant was used, while Freund’s incomplete adjuvant was used in the latter three injections. Blood samples were obtained before each booster injection for determ ination of serum antibody titer. Serum was separated from blood samples. The anti-PreS sera were purified using Protein A Sepharose 4B Fast Flow affinity adsor ptiongel column and was stored at -20 °C for later use.
The ELISA plate was coated with purified rabbit anit-PreS polyclonal antibody at a concentration of 10 mg/L at 4 °C stored overnight. After blockage with 1% BSA and dilution, the plate was vaccum-dried. then it was stored at 4 °C for later use. When it is used, test blood sample (50 μL) was added and incubated at 37 °C for 30 min. After washing, the enzyme -labelled anti-HBs monoclonal antibody was added and incubated at 37 °C for 30 min after washing, OPD color developer was added to develop the color by protecting from light at 37 °C for 10 min, then 2 mol/L H2SO4 was added to terminate the reaction. The 492 value was determined using DG3022 Enzyme Labelling Instrument. When the D value of the test sample was ≥ the average positive value × 0.08, it was taken as positive, otherwise, as negative.
The PreS-positive sera were diluted with serum-diluting solution containing purified rabbit anti-PreS antigen polyclonal antibody ( 20 mg/L ). After incubation at 37 °C for 1 h, the diluted sera were placed at 4 °C overnight. The n 100 μL of the sera were taken out and were added to the ELISA plate coated with rabbit anti-PreS1 antibody. After that the plate was incubated at 37 °C for 30 min. The rest steps were carried out by following the routine. The neutralizing inhibition rate ≥ 50% was taken as positive.
Math 1
Using pQe8 expression vector, the vector pQePreS1/2 expressing HBV ayw subtype including PreS1 and PreS2 gene fragments was constructed. After incubation, PreS1/2 protein with the relative molecular mass of 22000 (including three parts-PreS1:10-108/preS2:1-55/S1-22 ) was expressed. There were six histidine residues binding to the N-terminal of the product, the quantity of the recombinant protein expressed amounted to about 10% of the whole bacteria. The PreS1/2 existed mainly in inclusion bodies. The inclusion bodies were dissolved by denaturant and were purified on Ni-NTA agarose column. A purity over 95% was achieved. From 1 L cultured bacteria, 10 mg purified prote in could be obtained. Then the denaturant was removed from the purified protein in the course of renaturation.
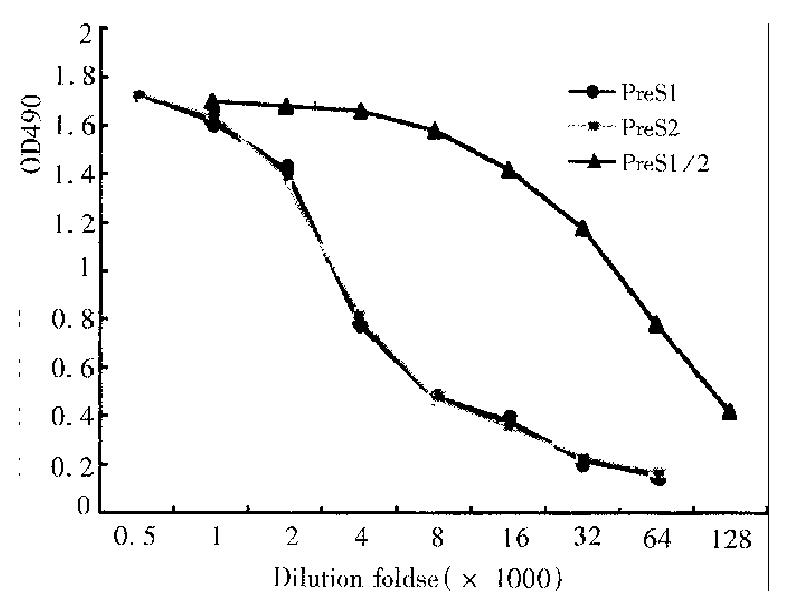
Using routine methods, rabbits were immunized with renatured recombinant PreS1/2 antigen and the titers of their immune sera against the recombinant prote in were determined by ELISA. Rabbit sera of blood samples taken before immunization served as the negative control (D value < 0.01 ). The titer of antibody directing against PreS1/2 antigen in rabbit sera reached 1:128000, 1:512000, respectively on 40 d, 60 d after first immunization. In order to identify the immunogenicity of different components of the recombinant PreS1/2 antigen, recombinant PreS2 (PreS2: 1-55/S:1-9) and PreS121-47 synthetic polypeptides were also used in determination of the serum titer on d40 ( Figure 1 ), and the result indicated in the immune sera the titers of antibodies directing against PreS1 polypeptide and PreS2 antigen were similar.
Using purified anti-PreS1/2 polyclonal antibody a double antibody sandwic h ELISA method for detecting PreS1/2 antigen was established. The anti-ser a of rabbits immunized with PreS1/2 were purified by using Protein A Sep harose 4B Fast Flow affinity adsorption gel column. Then 10 mg/L of the purified antibody was used to coat ELISA plate to adsorb HBV viral particles present in the sera and PreS1/2 antigen on the subunit particles. The specifically absorbed particles were detected with enzyme labeled anti-HBs antibody. This method was used in determination of purified Dane particle sera. Along with multiple proportional dilution, the D values of reaction wells decreased successively and the dilution titer of the sera could be up to 1:2048, corresponding to detection of 5000 HBV viral particles per mL. Purified Dane particle sera and 99 hepatitis C patients’positive sera were detected and the result of the former was positive, whereas that of the latter was negative. Neutralization of PreS antigen-positive patients’ sera with purified rabbit anti-PreS antibody resulted in competitive inhibition all rates reached over 80%.
Judged by intra-and inter-assay coefficients of variation, the precision of the detection method for PreS/2 antigen was evaluated. The intra-assay coefficient of variation was revealed by choosing PreS antigen-positive sera with high, medium and low values one each and by paralleled determination of 10 well s simultaneously each. The inter-assay coefficient of variation was demonstrated by determination of the above three sera for 10 times each at different time. The intra-assay coefficients of variation of the high, medium, low PreS antigen-positive sera were 3.8%, 4.5% and 13.3%, respectively; while the inter-assay coefficients of variation were 2.56%, 3.17% and 10.5%, respectively. These results indicate that precision has reached the requirement of Ministry of Public Health of China for intra and inter-assay coefficients of variation for diagnostic reagents (the national standard is ≤ 15%). Detection rates of PreS1/2 antigen in various populations of people, in sera of 200 normal blood donors, PreS1/2 was not positively detected. The detection rates of PreS1/2 antigen in sera of patients at different phases of HBV infection were listed in Table 1.
| Phase of infection | Number of cases | Positive rate(%) | ||
| Negative PreS1/2 | Positive PreS1/2 | Total | ||
| HBsAg(+)HBeAg(+)Anti-HBc(+) | 40 | 260 | 300 | 87 |
| HBsAg(+)Anti-HBe(+)Anti-HBc(+) | 50 | 142 | 192 | 74 |
| Anti-HBs(+) | 24 | 0 | 24 | 0 |
The PreS domain of HBV protein locates on the surface of HBV particle and can induce protective antibody in human body and in mouse. In the literature it is reported that the elimination of PreS antigen from serum patients with acute hepatitis B can foresee the disappearance of clinical symptoms. For patients with chronic hepatitis B, persistent existence of PreS in serum indicates progression of the disease course. In the meantime, monitoring PreS1/2 antigen has also important significance for evaluation of curative effect of antiviral therapy in the se patients.
Most investigators have established detection methods for PreS antigen by using anti-PreS monoclonal antibody. This study used highly expressed in Escherichia coli and purified HBV PreS antigens, which cover the whole PreS2 domain and the most parts of PreS1 domain. Three experimental results indicate that the re comb inant PreS antigen is also a good immunogen in rabbit. In rabbit anti-PreS sera, the titer of antibody directing against PreS1:21-47 basically equal to the o f antibody directing against PreS2: 1-55. A method of detecting PreS antigen established by using purified rabbit polyclonal antibody is predicted to be able to determine simultaneously PreS1 and PreS2 antigens in serum of patient. By using this method, we have detected preliminarily PreS antigen in HBeAg positive and anti-HBeAg positive and the results show that the detection frequency of PreS antigen is relatively high in both situations. Statistical analysis indicates that the presence of PreS antigen in serum is independent of the existence of HBeAg ( χ2 = 12.59, P < 0.01).
Edited by Xie-Ning Wu
Proofread by Qi-Hong Miao
| 1. | Delfini C, Colloca S, Taliani G, Mazzotta F, D'Agata A, Buonamici C, Stroffolini T, Carloni G. Clearance of hepatitis B virus DNA and pre-S surface antigens in patients with markers of acute viral replication. J Med Virol. 1989;28:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Petit MA, Zoulim F, Capel F, Dubanchet S, Dauguet C, Trepo C. Variable expression of preS1 antigen in serum during chronic hepatitis B virus infection: an accurate marker for the level of hepatitis B virus replication. Hepatology. 1990;11:809-814. [PubMed] |
| 3. | Neurath AR, Seto B, Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine. 1989;7:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Galán MI, Tomás J, Bernal MC, Salmerón FJ, Maroto MC. Evaluation of the pre-S (pre-S(1)Ag/pre-S(2)Ab) system in hepatitis B virus infection. J Clin Pathol. 1991;44:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Coursaget P, Buisson Y, Bourdil C, Yvonnet B, Molinié C, Diop MT, Chiron JP, Bao O, Diop-Mar I. Antibody response to preS1 in hepatitis-B-virus-induced liver disease and after immunization. Res Virol. 1990;141:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |