Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.488
Revised: September 2, 1999
Accepted: September 22, 1999
Published online: December 15, 1999
AIM: To investigate the activation, expression of c-src gene and its role in the carcinogenetic process of human cardia adenocarcinoma (CA).
METHODS: Fifty-six cases of CA, 34 cases of normal, 36 cases of protife rative epithelia adjacent to carcinoma, and 20 cases of lymph node metastases of CA were studied for PP60c-src, the expression product of c-src gene immunohistochemically by using the specific monoclonal antibody, Mab327.
RESULTS: The positive rates of PP60c-src in the normal ep ithelia, protiferative epithelia, CA and lymph node metastases were 29.4% (10/34), 94.4% (34/36), 71.4% (40/56) and 60.0% (12/20), respectively, among them, the differences of the positive rates were statistically significant ( P < 0.01). The expression levels of PP60c-src in CA and proliferative epithe lia were significantly higher than that in the normal epithelia (P < 0.01). The PP60c-src positive rates in the papillary, tubular, poorly differentiated and mucous adenocarcinoma were 75.0% (6/8), 81.8% (18/22), 50.0% (10/20) and 100.0% (6/6), respectively, whereas those of tubular and mucous adenocarcinomas were significantly higher than those of papillary and poorly differentiated adenocarcinomas (P < 0.05), and the PP60c-src expression levels of tubular and mucous adenocarcinomas were also significantly higher than those of papillary and poorly differentiated adenocarci nomas(P < 0.01).
CONCLUSION: The activation and expression of c-src gene are associated with the initiation and development of human CA; the protein amount of PP60c-src increased during the process of carcinogenesis; and PP60c-src expression is also related to lymph node metastases.
- Citation: Wang XJ, Yuan SL, Xiao L, Wang XH, Wang CJ. Expression of c-src gene in the carcinogenesis process of human cardia adenocarcinoma. World J Gastroenterol 1999; 5(6): 488-491
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.488
There is a general tendency in gastric cancer that the incidence rate of cardia adenocarcinoma (CA) is increasing steadly, and cancer of the distal stomach is decreasing proportionately. The biological and epidemiological features of CA are distinct from those of the distal stomach, and the underlying cause remained unelucidated[1-3]. PP60c-src is the product of c-src gene pos sessing the activity of tyrosine kinase. Increased expression of c-src gene had been reported in some human sarcoma and cancers of breast[4], esoph agus[5], stomach[6] and colon[7], and the activation and expression of c-src gene might be associated with the initiation and devel opment of some cancers. Our previous study showed the activation and expression of c-src gene was associated with the development and differentiation of esophageal squamous cell carcinomas[8], we believed that s imilar changes might occur in cancer of cardia, therefore, the following study was carried out.
All 56 cases of CA samples were collected from the CA patients surgically treated in Yanting Institute of Cancer Prevention of Sichuan Province. All tissue specimens were routinely processed, formalin-fixed and paraffin-embedded, at least 2 serial paraffin sections of 4 μm-6 μm thickness were made, one was stained with hematoxylin and eosin (HE) and the other was use d for PP60c-src protein detection by immunohistochemical staining.
The monoclonal antibody Mab327 (mouse IgG) was kindly given by the Molecular Pathology Department of Nagoya University, Japan; Streptavidin-Peroxidase Immunohistochemical Staining Kit (Zymed USA) was purchased from Fuzhou Maxim Biotech, Inc.
PP60c-src protein was detected immunohistochemically with LSAB method according to the manufacturer’s instructions with slight modification. Briefly, the tissue sections were deparaffinized and rehydrated through graded alcohols, and digested with trypsin. Then, endogenous peroxidase activity was blocked with 3% H2O2, and after treatment with normal serum, the sections were incubate d with Mab 327 at a dilution of 1:100 overnight at 4 °C, with biotinylated second antibody 20 min, and with streptavidin peroxides 30 min at room temperature. Subsequently, the sections were subjected to color reaction with 0.02% 3, 3-diaminobenzidine tetrahydrochloride containing 0.005% H2O2 in PBS (pH 7.4), and were counterstained with hematoxylin lightly. In each staining run, a known PP60c-src positive sample was added as positive control, and a section of the same sample was incubated with PBS instead of Mab327 as negative control.
Histopathological diagnosis for CA and related lesions were made, and the histological types of CA were examined by 2 expierenced pathologists according to the given criteria[8].
The immunostaining results of PP60c-src were analyzed according to the kn own criteria[5]. The percentages of positive PP60c-src cell in CA and related lesions were assessed and scored as follows: negative ( - ), < 25% ( + ), 25%-50% ( ++ ), > 50% (+++); The intensity of staining in PP60c-src positive cytoplasm and/or cell membrane were compared with the negative control and scored as follows: negative (-), weak (+), moderate (++) and strong (+++).
Among the 56 cases of CA samples, there were 34 normal epithelia adjacent to cancer, 36 proliferative epithelia adjacent to cancer, 56 adenocarcinomas including papillary ( 8/56 ), tubular ( 22/56 ), poorly differentiated ( 20/56 ), mucous adenocarcinomas ( 6/56 ), and 20 CA with lymph node metastases.
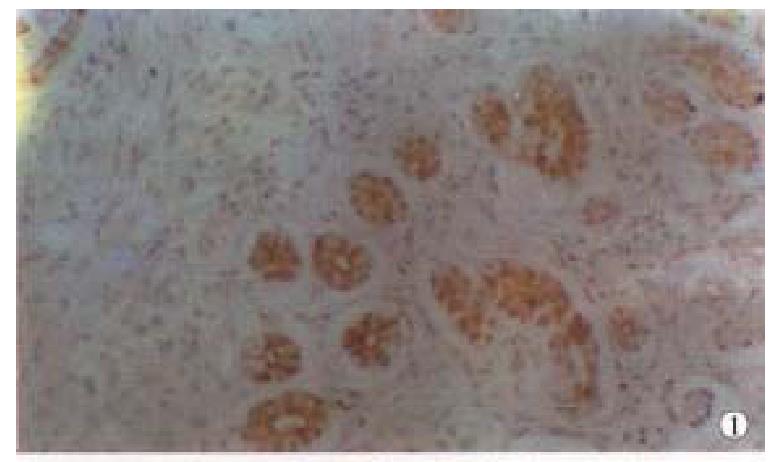
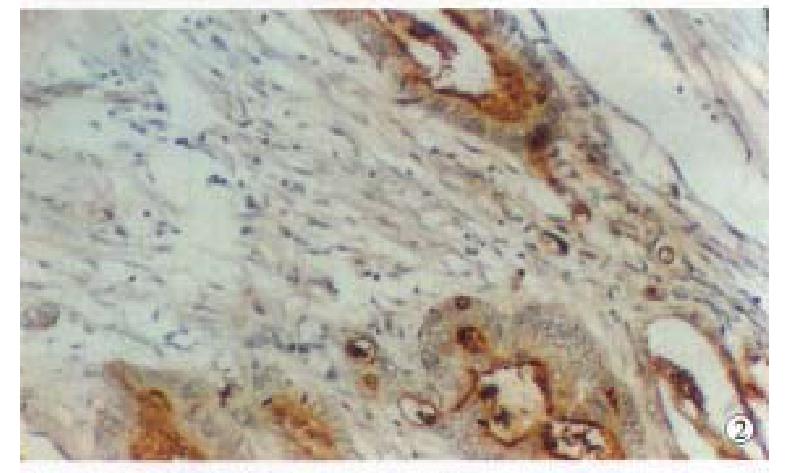
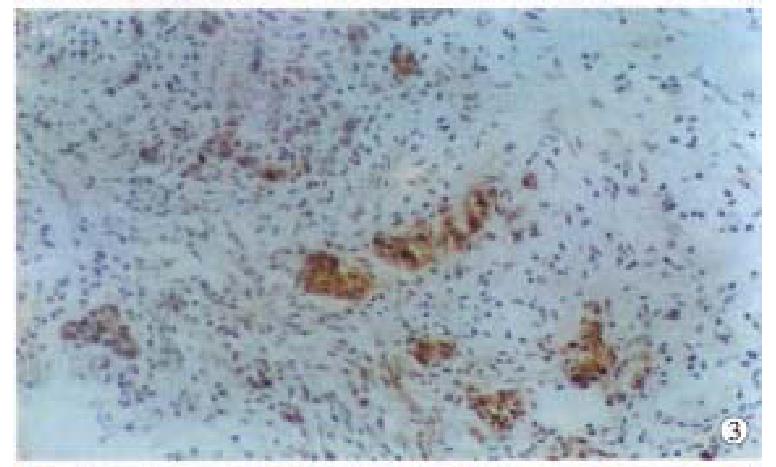
Positive PP60c-src protein cells showed brown staining in their cytoplasm s and cell membranes, no positive staining was found in the negative controls. The intensity of staining varied with different lesions and different histologic types. Positive staining of PP60c-src was localized in apex of glandular epithelial cells (Figure 1); in papillary and tubular adenocarcinomas, positive staining was distributed along the papillary margin or glandular lining, both the cytoplasms and cell membranes were positively stained but that of cell membranes were stronger than those in cytoplasms (Figure 2); the positive staining in poorly differentiated adenocarcinomas were evenly distributed in cytoplasms and cell membranes (Figure 3); and in the mucous adenocarcinomas, the positive PP60c-src staining was unevenly distributed as micromasses.
The positive rates of PP60c-src expression in CA, proliferative and norma l epithelia adjacent to cancer were 71.4% (40/56), 94.4% (34/36) and 29.4% (10/34), respectively (Table 1). The positive rates of the former two were higher than that in the latter (P < 0.01), and the positive staining in tensities in CA and proliferative epithelia were also stronger than that in the normal epithelia (P < 0.01).
The positive rates of PP60c-src in papillary, tubular, poorly differentia ted and mucous adenocarcinoma were 75.0% (6/8), 81.8% (18/22), 50.0% (10/20), and 100.0% ( 6/6 ), respectively ( Table 2 ) and the positive rates in tubular and mucous adenocarcinomas were higher than those in poorly differentiated or papillary adenocarcinomas (P < 0.05); the percentages of high PP60c-srcªª expression (+ + - + + +) in tubular and mucous adenocarcinoma were 63.6% (14/24) and 100.0% (6/6), respectively; while that in papillary and poorly differentiated ones were all low (+), there were significant differences between them (P < 0.01).
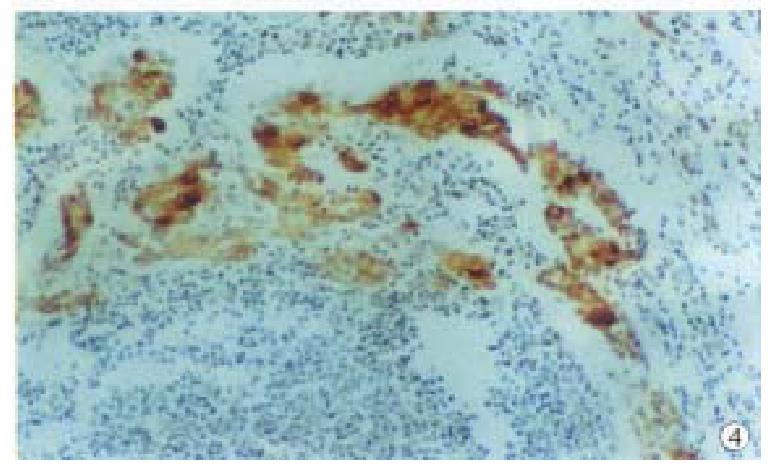
The positive rate of PP60c-src expression was 60.0% (12/20) in 20 case s of metastatic lymph node (Table 1), among them, the positive ones in papillary, tubular, poorly differentiated and mucous adenocarcinomas were 2/2, 2/4, 6/12 and 2/2, respectively; the expression intensity of P P60c-src in CA with lymph node metastases was the same as those without (Figure 4).
PP60c-src is a phosphorylated cytoplasmic protein encoded by c-src gene, having the activity of tyrosine kinase. The expression of PP60c-src can be found in many normal cells, which plays important roles in the regulation of cell proliferation, differentiation and transformation[4] Recent studies indicated that the increase in the amount of PP60c-src protein and kinase activity was associated with initiation and development of some human neoplasms, and the activation and increase of expression were one of the factors for cancer initiation[4-8]. In this study, the expressions of PP60c-src in CA, proliferative epithelia and normal epithelia were 71.4% (40/56 ), 94.4% ( 34/36 ) and 29.4% (10/34), respectively and the positive rates of PP60c-src in CA and proliferative epithelia were much higher than that in the normal epithelia (P < 0.01). Jankowiski et al analyzed the expression product of c-src gene in 15 cases of esophageal adenocarcinoma and 15 cases of Barrett’s esophageal epithelia immunohistochemically, the positive rates were 20% (3/15), suggestin that the expression of c-src gene is related to the development of esophageal adenocarcinoma. Therefore, the results of t his study indicated that the activation and expression of c-src gene might be associated with the initiation and development of CA. However, the high expression of PP60c-src in the proliferative epithelia might be associated with the proliferation of glandular epithelial cells, occurring in the aged rats[9]. The low PP60c-src expression in some normal epithelia adjacent to cancer might be explained by the fact that there had been PP60c-src expression in the normal epithelia related to the initiation of cancer[5-8]. On the other h and, it indicated that the activation and expression of c-src gene might be an early event in the carcinogenesis of CA.
Athough it was well known that the activation and expression of c-src gene were associated with the initiation of some human neoplasms. The results obtained mainly from biochemical assay by measuring the protein amount and kinase activity of PP60c-src varied with the methodology. Most of the studies reported that PP60c-src protein kinase activity increased in the cancer cell lines and cancer tissues from cancers of stomach, colon, lung and kidney, etc., but compared with those of normal tissues related to cancer, no difference of PP60c-src protein amount was found, the increase in PP60c-src protein kinase activity could not be explained by the increase of protei n expression encoded by c-src gene[6,10]. In the present study, PP60c-src protein was detected by using the specific monoclonal antibody Mab 327, immunohistochemically, the high level of PP60c-src expressions ( + + - + + +) in CA and proliferative epithelia were 35.7% and 77.8%, respectively, a low PP60c-src expression (+) was found in some normal epithelia adjacent to cancer, the difference of expression intensity was significant statistically (P < 0.01). The results of this study suggested that the protein amount of PP60c-src expression was increased in carcinogenesis of CA.
With regard to the relationship between expression product of c-src gene, PP60c-src and the differentiation of cancer cells, there was no consensus in this aspect[6,8,9,11]. Fanning et al[1] reported a high l evel of c-src expression in well differentiated bladder cancers, and pro posed that PP60c-src protein and kinase activity were associated with the differentiation of epithelial cells of urinary tract and grade I-II bladder carcinomas. However, Takekura et al[6] could not find the differen ce of PP60c-src kinase activity between well and poorly differentiated ga stric carcinomas. In this study, PP60c-src positive rates varied in different histological types of CA; the positive rates in mucous adenocarcinomas ( 100.0%, 6/6 ) and tubular adenocarcinomas ( 81.8%, 18/22 ) were higher than those in papillary (75.0%, 6/8) and poorly differentiated types (50.0%, 10/20), the difference being significant statistically ( P < 0.05 ). And the high level of expressions (++ - +++) in mucous and tubular adenocarcinomas were 100.0% (6/6) and 63.6% ( 14/18 ), respectively, only low expression (+) was found in papillary and poorly differentiated adenocarcinomas, the differences of expression level were also significant statistically (P < 0.01). These results suggested that the expression level of PP60c-src was associated with the differ entiation and histological types of CA, high PP60c-src expressions in mucous and tubular adenocarcinomas might be related to their well differentiation and other biologic behaviors.
The relationship between the PP60c-src expression with increase in kinase activity and metastatic colon carcinomas had been reported already[12,13], but there was no report of detection of PP60c-src protein in lymph no de metastases by immunohistochemical staining. Talamonti et al[12] discovered increase in PP60c-src kinase activity, in the stages o f polyps and primary carcinomas. Termuhlen et al[13] reported that P P60c-src kinase activity in hepatic metastases of colorectal carcinoma in creased by 2.2 folds, and in extrahepatic metastases increased by 12.7 folds, while compared with that in normal mucosa, PP60c-src kinase activity in the hepatic metastases of non-colorectal carcinomas increased only slightly. In this study, PP60c-src protein was detected in the lymph node metastases o f CA, the positive rate was 60.0% (12/20), the expression level of PP60c-src was equal to that of the same primary adenocarcinoma. The results of this study suggested that PP60c-src expression is associated with the lymph node metastases of CA, which deserved further investigation.
Project supported by the grant of West China University of Medical Scien ces, No.L293015
Edited by Xie-Ning Wu
Proofread by Qi-Hong Miao
| 1. | Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer. 1990;62:440-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 279] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1149] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 3. | Sampliner RE. Adenocarcinoma of the esophagus and gastric cardia: is there progress in the face of increasing cancer incidence. Ann Intern Med. 1999;130:67-69. [PubMed] |
| 4. | Jacobs C, Rübsamen H. Expression of pp60c-src protein kinase in adult and fetal human tissue: high activities in some sarcomas and mammary carcinomas. Cancer Res. 1983;43:1696-1702. [PubMed] |
| 5. | Jankowski J, Coghill G, Hopwood D, Wormsley KG. Oncogenes and onco-suppressor gene in adenocarcinoma of the oesophagus. Gut. 1992;33:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Takekura N, Yasui W, Yoshida K, Tsujino T, Nakayama H, Kameda T, Yokozaki H, Nishimura Y, Ito H, Tahara E. pp60c-src protein kinase activity in human gastric carcinomas. Int J Cancer. 1990;45:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989;83:2025-2033. [PubMed] |
| 8. | Wang X, Wang C, Huang G, Xiao H. [A study of c-src gene express product pp60c-src in esophageal carcinoma]. Huaxi Yike Daxue Xuebao. 1995;26:197-201. [PubMed] |
| 9. | Majumdar AP, Tureaud J, Relan NK, Kessel A, Dutta S, Hatfield JS, Fligiel SE. Increased expression of pp60c-src in gastric mucosa of aged rats. J Gerontol. 1994;49:B110-B116. [PubMed] |
| 10. | Cartwright CA, Meisler AI, Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci USA. 1990;87:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 212] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Fanning P, Bulovas K, Saini KS, Libertino JA, Joyce AD, Summerhayes IC. Elevated expression of pp60c-src in low grade human bladder carcinoma. Cancer Res. 1992;52:1457-1462. [PubMed] |
| 12. | Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 315] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Termuhlen PM, Curley SA, Talamonti MS, Saboorian MH, Gallick GE. Site-specific differences in pp60c-src activity in human colorectal metastases. J Surg Res. 1993;54:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |