Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.432
Revised: June 20, 1999
Accepted: September 14, 1999
Published online: October 15, 1999
- Citation: Wang XT, Zhuang H, Song HB, Li HM, Zhang HY, Yu Y. Partial sequencing of 5'non-coding region of 7 HGV strains isolated from different areas of China. World J Gastroenterol 1999; 5(5): 432-434
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/432.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.432
Although sensitive tests for detection of known hepatitis viruses are available, the etiology of 10%-15% post-transfusion and community-acquired hepatitis cases has remained undefined. It suggests the existence of unknown causative agents associated with the disease. GBV-C and HGV were newly discovered as putative non-A to E hepatitis viruses reported by Simons[1] and Linnen[2] independently. However, the sequence homology analysis of the two strains revealed that they are different isolates of the same virus. HGV is a positive-strand RNA virus with an entire genome of 10kb which contains a continuous open reading frame (ORF) encoding a viral polyprotein. The structural region (C, E1 and E2) is located at the N-terminal, while the non-structural region (NS2, NS3, NS4A/B, NS5A/5B) is situated at the C-terminal. The long ORF is preceded by a 5’ untranslated sequence and followed by a 3’ untranslated sequence. Our previous report has confirmed the existence of HGV infection in China[3]. There is evidence that the gene of hepatitis C virus (HCV) is hypervariable in different areas[4-8]. The variability of HCV is also found in the same strain of the virus. HGV and HCV are classified in the same genus of the flaviviridae family. So it is of great significance to clarify the geographical distribution of HGV genotypes in the world[9]. In this study, the partial sequences of 5'non-coding region of 7 HGV strains isolated from different areas of China were analyzed and compared with GBV-C (U36380) and HGV (U44402) reported from the United States.
Seven HGV RNA positive sera tested by RT-PCR were collected from blood donors of Beijing, Jiangsu, Anhui, Liaoning, Hebei Provinces, and Guangxi Zhuang and Xin jiang Uighur Autonomous Regions.
According to the nucleotide sequence of 5'non-coding region of a Chinese HGV strain, the primers for RT nPCR were designed using the software of OLIGO 5.0. They were as follows: S1 5’GGTGGTGGATGGGTGATGAC3’; A1 5’CCGAAGGATTCTTGGGCTAC3’; S2 5’GCTGGTAGGTCGTAAATC3’; A2 5’ACTGGTCCTTGTCAACTC3’.
HGV RNA extraction, HGV cDNA synthesis and PCR procedure were performed by the methods described previously[3]. All the PCR products were cloned into the pGEMT vector (Promega, Madison, WI), and positive clones were identified. The PCR products were purified and sequenced bidirectionally using the dideoxynucleotide chain termination method. The HGV cDNA sequences were analyzed with a DNA sequencer (ABI PRISM 377 DNA Sequencer, Perkin-Elmer Cetus).
The positive rates of anti-HGV varied from 1.2% (35/2916) to 5.4% (49/907) in blood donors and 42.9% (15/35) 75.5% (37/49) of anti-HGV positive sera were also HGV RNA positive.
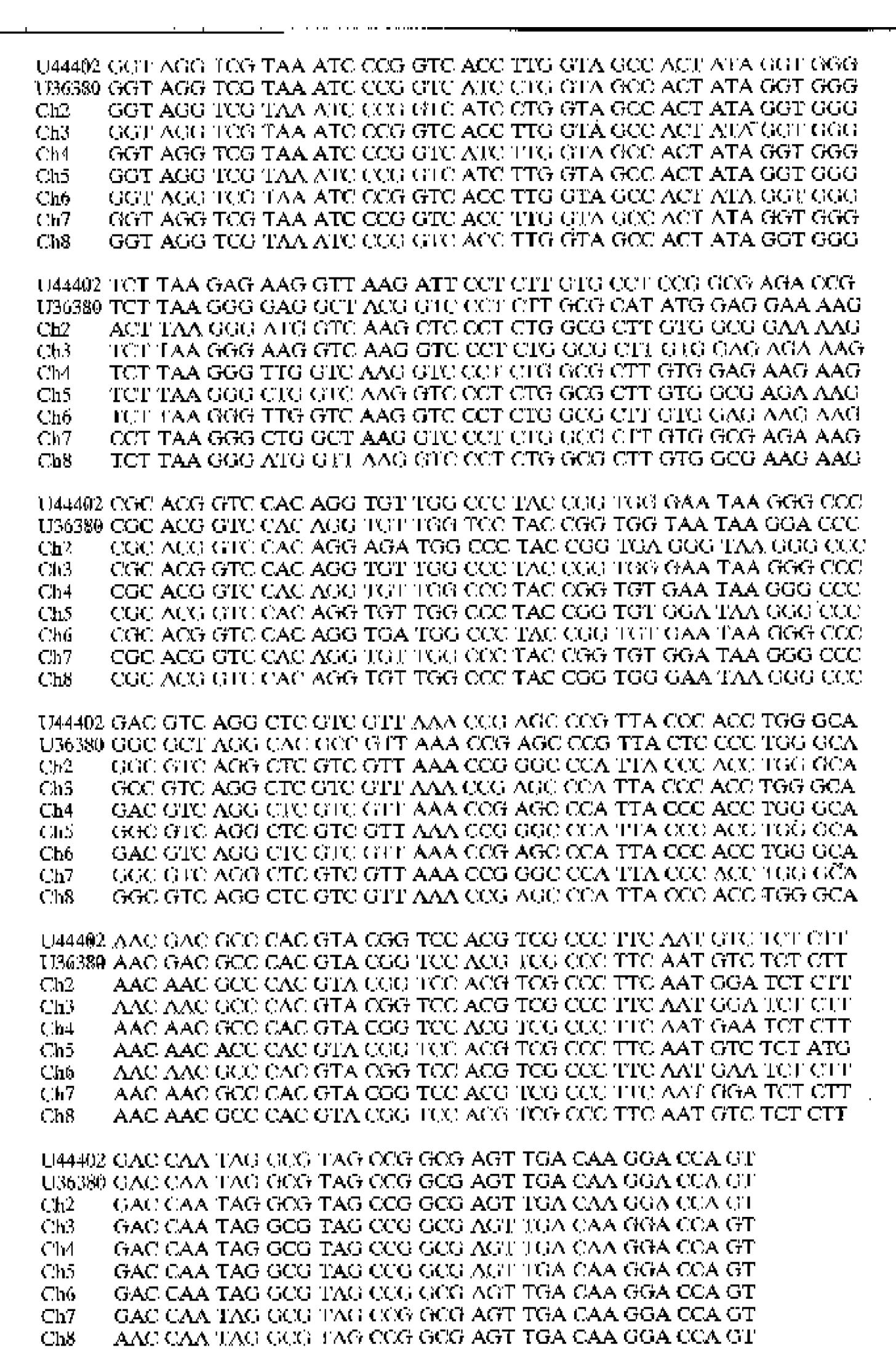
The partial nucleotide sequences of the 5’non-coding region of 7 HGV strains isolated from blood donors of Beijing, Jiangsu, Anhui, Liaoning, Hebei Provinces, and Guangxi Zhuang and Xinjiang Uighur Autonomous Regions, China were analyzed and compared with GBV-C (U36380) and HGV (U44401) (Figure 1).
The nucleotide homology of the 5'non-coding region of 7 Chinese HGV strains was 85.92%, 88.26%, 88.26%, 85.45%, 86.85%, 85.92% and 88.26%, respectively, as compared with the African strain GBV-C (U36380). It was 86.85%, 92.02%, 86.67%, 89.02%, 89.67% and 91.55%, respectively, as compared with the A merican strain HGV (U44402). The homology of nucleotide sequences was 92.02%-97.18% among the 7 Chinese HGV strains (Table 1).
| HGV strains | Homology of the nucleotides (%) | ||||||||
| U36380 | U44402 | Ch2 | Ch3 | Ch4 | Ch5 | Ch6 | Ch7 | Ch8 | |
| U36380 | 100.0 | ||||||||
| U44402 | 87.23 | 100.0 | |||||||
| Ch2 | 85.92 | 86.85 | 100.0 | ||||||
| Ch3 | 88.26 | 92.02 | 93.42 | 100.0 | |||||
| Ch4 | 88.26 | 86.67 | 92.96 | 96.24 | 100.0 | ||||
| Ch5 | 85.54 | 89.20 | 92.96 | 93.90 | 94.37 | 100.0 | |||
| Ch6 | 86.85 | 89.67 | 92.96 | 96.24 | 99.06 | 93.43 | 100.0 | ||
| Ch7 | 85.92 | 89.67 | 94.84 | 97.18 | 95.31 | 96.71 | 95.31 | 100.0 | |
| Ch8 | 88.26 | 91.55 | 92.02 | 95.30 | 95.31 | 93.70 | 95.31 | 94.37 | 100.0 |
HGV is transmitted parenterally, and the infection seems not to cause significant hepatic damage as hepatitis viruses A E do. Although transmission through blood or parenteral exposure is well documented for HGV, little is known about its prevalence in blood donors of China. This study shows that the prevalence rate of anti-HGV ranged from 1.2% to 5.4% in the population of different areas of China. The data indicate that the HGV infection is widely spread in the different areas of China. The nucleotide homology of the 5'non-coding region among the 7 Chinese HGV strains was 92.0%-97.2%. However, the identity of these 7-Chinese strains was 85.9%-92.0% at the nucleotide level as compared with the African strain of GBV-C (U36380) and the American HGV strain (U44402). The data suggest that the Chinese HGV isolates belong to a new group which is different from the African and American strains reported by Simons[1] and Linnen[2]. The divergence of nucleotide sequences among Chinese HGV strains shows the correlation between HGV variation and the geographical locations.
The homology of NS3 nucleotide sequences of the 3 Chinese HGV strains reported previously by our group[3] was 92.48%, 89.09% and 85.34%, respectively with GBV-C (U36380), and 89.09%, 85.34% and 85.34% with HGV (U44402). It is very close to the homology of the 5'non-coding region of 7 Chinese HGV strains with GBV-C (U36380) and HGV (U44402), indicating that the NS3 region may not be the site of immune selection.
We are grateful to FAN Jin-Shui, LI Kui and ZHU Yong-Hong for their helpful advices.
Edited by Ma JY
| 1. | Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, Desai SM, Mushahwar IK. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564-569. [PubMed] |
| 2. | Linnen J, Jr JW, Zhang-keck ZY, Fry KE, Krawczynski KZ, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505-509. |
| 3. | Wang XT, Zhuang H, Li HM, Fan JS, Qi ZB, Liu G. Detection of GBV-C: infection and sequencing of partial gene of a Chinese strain of GBV-C. Zhonghua Weishengwuxue He Mianyixue Zazhi. 1996;16:263-265. |
| 4. | Lesniewski RR, Boardway KM, Casey JM, Desai SM, Devare SG, Leung TK, Mushahwar IK. Hypervariable 5'-terminus of hepatitis C virus E2/NS1 encodes antigenically distinct variants. J Med Virol. 1993;40:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Choo QL, Richman KH, Han JH, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr PJ. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1131] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 6. | Kato N, Ootsuyama Y, Tanaka T, Nakagawa M, Nakazawa T, Muraiso K, Ohkoshi S, Hijikata M, Shimotohno K. Marked sequence diversity in the putative envelope proteins of hepatitis C viruses. Virus Res. 1992;22:107-123. [PubMed] |
| 7. | Kato N, Hijikata M, Ootsuyama Y, Nakagawa M, Ohkoshi S, Shimotohno K. Sequence diversity of hepatitis C viral genomes. Mol Biol Med. 1990;7:495-501. [PubMed] |
| 8. | Okamoto H, Kojima M, Okada S, Yoshizawa H, Iizuka H, Tanaka T, Muchmore EE, Peterson DA, Ito Y, Mishiro S. Genetic drift of hepatitis C virus during an 8.2-year infection in a chimpanzee: variability and stability. Virology. 1992;190:894-899. [PubMed] |
| 9. | Schaluder GG, Dawson GJ, Simons JN, Pilot-Matias TJ, Gutierrez RA, Heynen CA, Knigge MF, Kurpiewski GS, Buijk SL, Leary TP. Molecular and serologic analysis in the transmission of the GB hepatitis agents. J Med Virol. 1995;46:81-90. [PubMed] |