Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.417
Revised: July 20, 1999
Accepted: September 24, 1999
Published online: October 15, 1999
AIM: To explore the relationship between the configuration changes of the nuclear matrix-intermediate filament system in cancer cell induced by retinoic acid and the malignant phenotypic reversion of cancer cells.
METHODS: The human gastric adenocarcinoma cell line MGc80-3 cells were induced with 10-6 mol/L retinoic acid and subcultured at cover slip strip and gold grids. The cells were treated by selective extraction method and prepared for whole mount electron microscopy observation. The samples were examined respectively with scanning and transmission electron microscope.
RESULTS: The nuclear matrix filaments and intermediate filament s in MGc80-3 cells were relatively few and scattered, not well-distributed and arranged irregularly. The nuclear lamina was ununiformly thick and compact, connected to the nuclear matrix filaments and intermediate filaments relaxedly. However, the two kinds of filaments were abundant and well-distributed, different in slender and thick form and interweaved into a regular network in the cells induced by 10-6 mol/L RA. The nuclear matrix filaments and intermediate filaments were connected closely by the thin and compact fiber-like lamina, and interlaced into a regular network throughout the whole cell region.
CONCLUSION: The NM-IF system in MGc80-3 cells had undergone a restorational change similar to those of normal cells after RA inducement. This alternation is an important morphological and functional expression to the malignant phenotypic reversion of cancer cells.
- Citation: Li QF. Effect of retinoic acid on the changes of nuclear matrix-intermediate filament system in gastric carcinoma cells. World J Gastroenterol 1999; 5(5): 417-420
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/417.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.417
The nuclear matrix is a fine network in the eukaryotic nucleus and plays an important role in maintaining nuclear morphology, DNA organization, DNA replication and gene expression. The morphological and functional alternations of the nuclear matrix have an important effect on cell proliferation and differentiation[1,2]. It has been shown in previous studies that there were dramatic differences in the configuration and protein composition of the nuclear matrix between cancer and normal cells[3,4]. Moreover, it has been demonstrated that some carcinogens or anti-cancer agents perform their function by affecting the nuclear matrix[5]. These results suggest that abnormal nuclear matrix is closely relevant to cell canceration. However, the alternations of nuclear matrix during the differentiation induced by cancer cells and its relation to the malignant phenotypic reversion of cancer cells are still poorly understood. To explore the correlation between the configurational changes of the nuclear matrix and the malignant phenotypic reversion of cancer cells, a study was made on the alternations of the nuclear matrix-intermediate filament (NM-IF) system in human gastric adenocarcinoma cell line MGc80-3 induced by retinoic acid (RA).
MGc80-3 cells were cultured in RPMI-1640 medium supplemented with 20% heat-inactivated fetal calf serum, and an appropriate amount of penicillin, streptomycin and kanamycin. The MGc80-3 cells were induced by the medium containing 10-6 mol/L all-trans-retinoic acid (RA) (purchased from Sigma Chemical Co.). Then, MGc80-3 cells and the cells treated with RA were seeded in small penicillin bottles with cover slip strip on which some gold grids covered with formvar and coated with carbon were sticked with polylysine, and grown in the normal medium or the medium containing 10-6 mol/L RA respectively. Cells were incubated at 37 °C in 5% CO2 atmosphere for 72 h.
The cells were selectively extracted as described by Capco[6]. MGc80-3 cells and the cells treated with RA were rinsed with D-Hank’s solution twice at 37 °C, and extracted by high ionic strength extraction solution (10 mmol/L PIPES, pH6.8, 250 mmol/L (NH4)2SO4, 300 mmol/L sucrose, 3 mmol/L MgCl2, 1.2 mmol/L PMSF, 0.5% Triton X-100) at 4 °C for 3 min. The extracted cells were rinsed in non-enzyme digestion solution (10 mmol/L PIPES, pH6.8, 50 mmol/L NaCl, 300 mmol/L sucrose, 3 mmol/L MgCl2, 1.2 mmol/L PMSF, 0.5% Triton X-100), and digested in digestion solution containing DNase I (400mg/L) and RNase A (400 mg/L) for 20 min at 23 °C. The samples were placed in high ionic strength extraction solution at 23 °C for 5 min. So far only the nuclear matrix-intermediate filament structure remained intact.
The NM-IF samples produced by selective extraction were prefixed in 2% glutaraldehyde (made in non-enzyme digestion solution) at 4 °C for 30 min. The samples were then rinsed with 0.1 mol/L sodium cacodylate buffer (pH7.4), postfixed in 1% OSO4 (made in 0.1 mol/L sodium cacodylate) at 4 °C for 5 min, dehydrated in ethanol series, replaced in isoamyl acetate, dried through the CO2 critical point. The cell samples attached to grids were examined with a JEM-100CX II transmission electron microscope (TEM), and the cell samples grown on cover slip strip were gilded in vacuum and examined with a HITACHI S-520 scanning electron microscope (SEM).
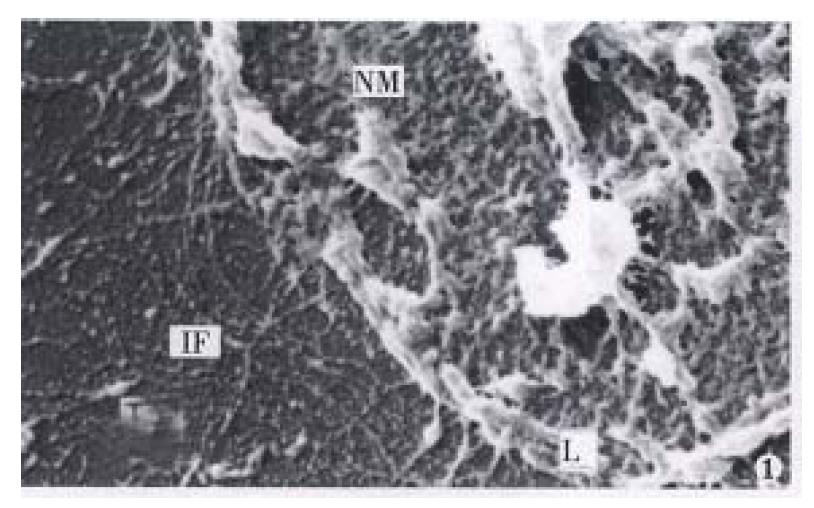
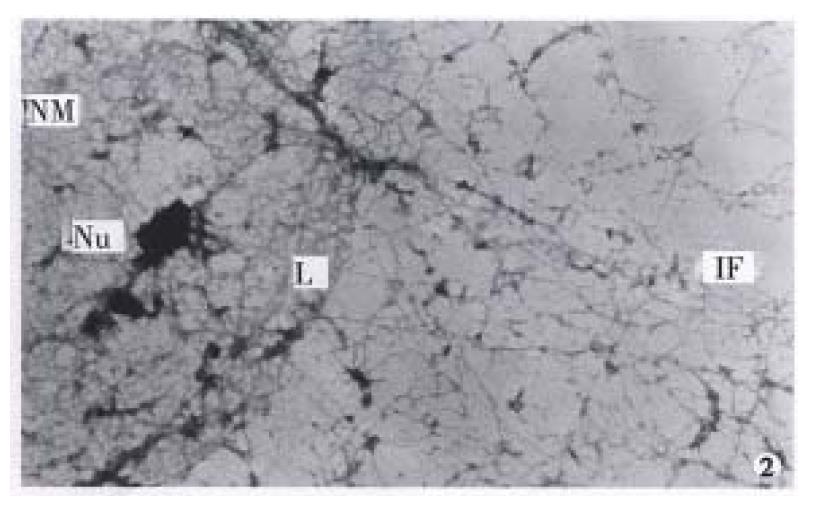
It was revealed by TEM and SEM that MGc80-3 cells and the cells induced by RA after selective extraction remained a filament network spreading all over the original cell region and structurally interlinked. In addition, the original nucleus region was maintained by the nuclear lamina and formed an interlinking and integrated NM-IF system (Figure 1, Figure 2, Figure 3). After induced treatment with RA, the changes of NM-IF system in MGc80-3 cells were observed.
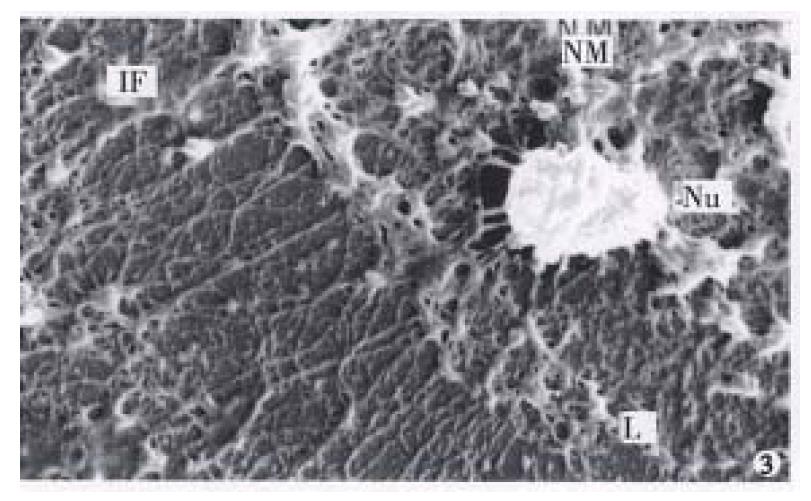
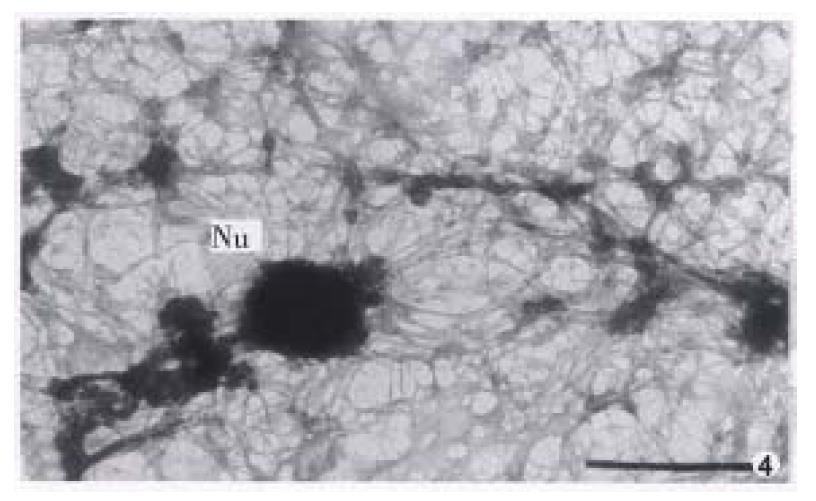
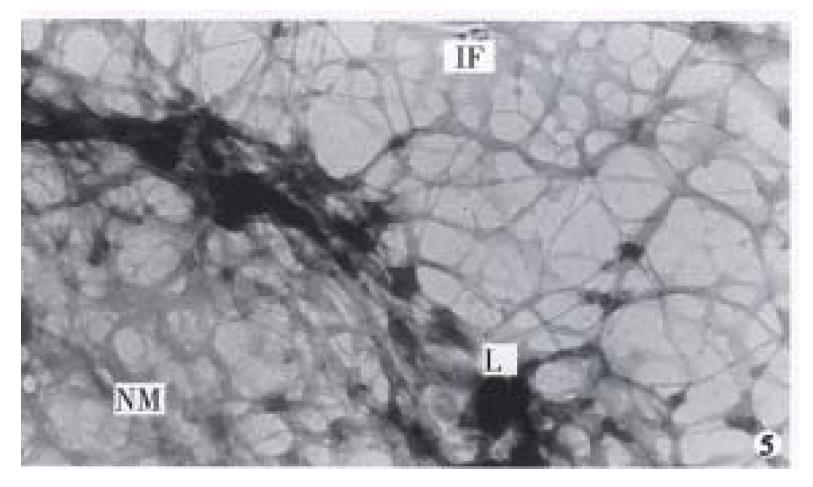
The nuclear matrix filaments in MGc80-3 cells were relatively few and scattered, not well-distributed and arranged irregularly within the nucleus region. The nuclear matrix filaments were short, and there were few single filaments while most of the nuclear matrix filaments were quite thick in bundle-like form and interweaved into an irregular network or in a flocculent structure. One or more residual nucleoli were usually observed within the nucleus region and maintained by a few of nuclear matrix filament bundles (Figure 1, Figure 2). However, in the MGc80-3 cells induced by RA, the nuclear matrix filaments were abundant and well-distributed, different in slender and thick form in the nucleus region. The nuclear matrix filaments were slender, in which the single filaments increased, and interweaved into a regular network. The nuclear matrix filaments or filament bundles were arranged radiately and connected to the residual nucleoli (Figure 3, Figure 4, Figure 5).
The nuclear lamina in MGc80-3 cells was ununiformly thick and compact. The inner nuclear lamina was nonected with some thick nuclear matrix filament bundles or thin and short filaments. It could always be seen that the intermediate filament bundle and the thin or thick filament of its branches were connected to and terminated on the outer nuclear lamina. Nevertheless, the nuclear matrix filaments and the intermediate filaments connected to the nuclear lamina were relatively few and scattered (Figure 1, Figure 2). The nuclear lamina in MGc80-3 cells induced by RA turned into a thin and compact fibroid structure. The inner nuclear lamina was connected closely with the nuclear matrix filaments, and many long and slender intermediate filament bundles were terminated directly on the outer nuclear lamina. Both the nuclear matrix filaments and the intermediate filaments connected to the nuclear lamina increased and appeared quite densely. It impelled the three parts to link up with each other more closely (Figure 3, Figure 5).
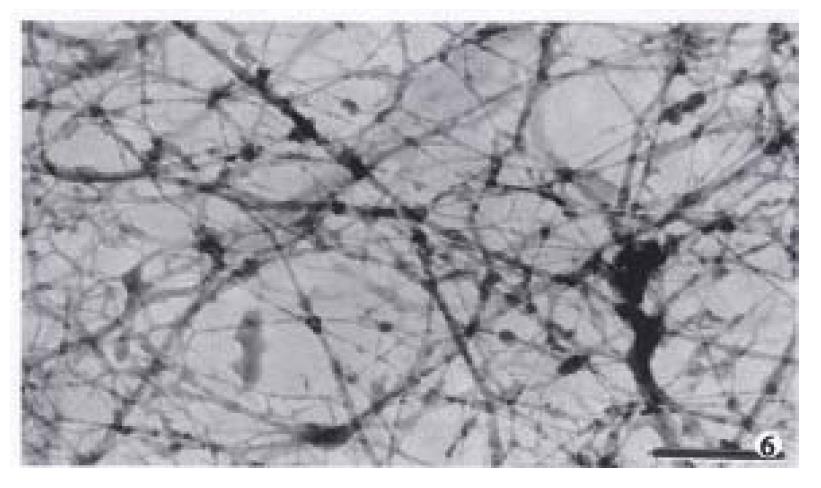
The amount of intermediate filaments in MGc80-3 cells was rather small. They were present mainly in the cytoplasm region around the nucleus and only a few in the peripheral region within cytomembrane, and were not well-distributed. The intermediate filaments in which single filaments were few, were chiefly in thick bundles or in strip-rope-like structure, and arranged irregularly (Figure 1, Figure 2). But they were abundant and well-distributed in the cytoplasm in MGc80-3 cells induced by RA. They spread from the region around nucleus and to the cellular edge. Quite a few single filaments were found in intermediate filaments which in terweaved with the slender intermediate filament bundles into a well-distributed and regular network throughout the cytoplasm region (Figure 3, Figure 5, Figure 6).
The abnormality of nuclear matrix is largely associated with the canceration of cell. Previous studies demonstrated that the nuclear matrix in cancer cells has undergone an irregular and abnormal configuration distinguished from that of normal cells[1,3], indicating that the nuclear matrix in cancer cells has some distinctive configuration characteristics which differed significantly from those of normal cells. The observation in this study displayed that the nuclear matrix filaments and intermediate filaments in MGc80-3 cells were relatively few, not well distributed, arranged irregularly, and the single filaments were few while filament bundles were plentiful. The thick nuclear lamina was not largely associated with the nuclear matrix filaments and intermediate filaments. It showed the typical configuration characteristics of nuclear matrix in malignant tumor cells. The NM-IF system in MGc80-3 cells induced with RA had undergone a significant change. The nuclear matrix filaments and intermediate filaments were abundant, well-distributed with the single filaments increased and differed in slender and thick form and inteweaved into a regular network. Meanwhile, the nuclear matrix and intermediate filaments were connected closely by the thin and compact fiber-like nuclear lamina and organized into an integrated network throughout the cell. The characteristics of this organized and integrated configuration of NM-IF system were significantly different from those of MGc80-3 cells but similar to those of normal cells of epithelial origin[6-7]. It demonstrated that RA could impel the NM-IF system in MGc80-3 cells to exert a reversional configuration alteration. In this regard, the restoration of the normal configuration of nuclear matrix is obviously an important morphological and functional expression to the malignant phenotypic reversion of gastric carcinoma cells.
The nuclear matrix plays a role not only in maintaining nuclear morphology as a framework within the nucleus, but in DNA replication and chromosomal construction. Consequently, the nuclear matrix can directly affect the cell division and proliferation[1,2,8]. It is obvious that the changes of nuclear morphology and the inhibition of DNA synthesis and cell proliferation in MGc80-3 cells induced by RA are associated closely with the configuration and functional alternation of the nuclear matrix. It can be concluded that these effects are resulted from the restoration of normal configuration of nuclear matrix in MGc80-3 cells induced by RA. In addition, the nuclear matrix plays an important influence upon regulating gene expression by acting on gene transcription, RNA processing and modifying, and directional transport[2,9]. Previous studies suggested that oncogene of cancer cells mainly existed in DNA sequences connecting to the nuclear matrix, and the transcription of oncogene couldn’t be underway until the oncogene was connected to the nuclear matrix[3,10]. It indicates that the nuclear matrix plays an important role in oncogene expression. Therefore, the restoration of normal configuration and function of nuclear matrix in gastric carcinoma cells induced with RA must have an important effect on regulating the expression of oncogene and tumor suppressor gene which are related to gastric carcinoma cells. For this reason, how to investigate the functional alternations of nuclear matrix in cancer cells during the induced differentiation will have a momentous significance in revealing the mechanism of cell canceration and reversion.
Edited by Wang XL
| 1. | Chen F, Zhai ZH. The nuclear matrix. Chin J Cell Biol. 1989;11:49-53. |
| 2. | Vemuri MC, Raju NN, Malhotra SK. Recent advances in nuclear matrix function. Cytobios. 1993;76:117-128. [PubMed] |
| 3. | Jiang DL. Functions of nuclear matrix and tumorigenesis. Foreign Med Sci: Mol Bio. 1989;11:18-21. |
| 4. | Getzenberg RH, Pienta KJ, Huang EY, Coffey DS. Identification of nuclear matrix proteins in the cancer and normal rat prostate. Cancer Res. 1991;51:6514-6520. [PubMed] |
| 5. | Fernandes DJ, Catapano CV. Nuclear matrix targets for anticancer agents. Cancer Cells. 1991;3:134-140. [PubMed] |
| 6. | Capco DG, Wan KM, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 322] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Fey EG, Wan KM, Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984;98:1973-1984. [PubMed] |
| 8. | Tsuchiya E, Miyakawa T. [Nuclear structure dynamics during the cell division cycle]. Tanpakushitsu Kakusan Koso. 1994;39:1575-1578. [PubMed] |
| 9. | Getzenberg RH. Nuclear matrix and the regulation of gene expression: tissue specificity. J Cell Biochem. 1994;55:22-31. [PubMed] |
| 10. | Chou RH, Churchill JR, Mapstone DE, Flubacher MM. Sequence-specific binding of a c-myc nuclear-matrix-associated region shows increased nuclear matrix retention after leukemic cell (HL-60) differentiation. Am J Anat. 1991;191:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |