Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.404
Revised: August 20, 1999
Accepted: September 24, 1999
Published online: October 15, 1999
AIM: To determine the activities of polysaccharide extracts from Flammulina velutipes (Curt. ex Fr.) Sing (FV), Lentinus edodes (LE) and Agaricus bisporus-Sing (AB) on the proliferation of human hepatoma SMMC-7721 cells in vitro and on mouse implanted S-180 tumors in vivo.
METHODS: The polysaccharide extracts were isolated from the fruit bodies of FV, LE and AB by the methods of hot-water extraction, Sevag’s removal of proteins, ethanol precipitation, trypsin digestion and ethanol fractional precipitation. Human hepatoma SMMC-7721 cells were treated with 50 mg/L polysaccharide extracts, and the mitosis index, mitochondria activity and cell proliferation were detected at different times in both control and experimental groups. The mice with S-180 implanted tumors were injected with the polysaccharide extracts at 24 mg/kg body weight for 9 d and the tumor weight was measured on the 15th day.
RESULTS: The mitosis index of hepatoma cells in vitro could be significantly decreased by treatment with the polysaccharide extracts from the three kinds of edible fungi (P < 0.005). The cell numbers and mitochondria activity of SMMC-7721 cells treated with polysaccharide extracts were lower than those in control groups (P < 0.005). The inhibition rates of polysaccharide extracts against implanted S-180 tumors in mice were 52.8%, 56.6% and 51.9% respectively compared with that in control groups.
CONCLUSION: The polysaccharide extracts from the three kinds o f edible fungi could inhibit not only the cultured malignant cells in vitro but also implanted S-180 tumor in vivo.
- Citation: Jiang SM, Xiao ZM, Xu ZH. Inhibitory activity of polysaccharide extracts from three kinds of edible fungi on proliferation of human hepatoma SMMC-7721 cell and mouse implanted S180 tumor. World J Gastroenterol 1999; 5(5): 404-407
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.404
The polysaccharides from edible fungi (e.g. LE[1], FV[2,3]) were macromolecular substances with strong antigenicity and were also verified to have antitumor activity against S-180 implanted tumor in mice in vivo and that from AB were shown to have anti-infection of virus and anticanceration in vivo. The references about the effects of polysaccharide extracts from edible fungi on cancer cells in vitro were very limited. In this report human hepatoma SMMC-7721 cells were used as a model to detect the anticancer activity of polysaccharide extracts from the three kinds of edible fungi (FV, LE and AB). The mitosis index, cell proliferation and mitochondria metabolism activity of SMMC-7721 cells were compared between the control group and polysaccharide extracts treatment groups. The antitumor activity of polysaccharide extracts from these three kinds of edible fungi against implanted S-180 tumor in mice in vivo was also observed.
RPMI 1640 medium is product of GIBCO; trypsin and MTT were from Sigma; the fruit bodies of FV, LE and AB were from cultivated products in Jinan. Mice with S-180 and Kunming male mice (22 g-25 g) were from Shandong Experiment al Animal Center; 24 and 96-well plates were from Costar.
Human hepatoma SMMC-7721 cells were obtained from the Shanghai Cell Bank of Chinese Academy of Sciences and maintained in our laboratory. The cells were grown as monolayers in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and incubated at 37 °C in the humidified incubator with 5% CO2/95% air.
The extraction and purification of polysaccharide extracts were modified according to the methods of Cao[3]. The fresh fruit bodies of the edible fungi were homogenized for three times, heated at 98 °C-100 °C for 5 h, centrifuged for 15 min with 4500 rpm. The supernatants were collected and the precipitation was extracted for another two times. All the supernatants were concentrated to proper volume and precipitated with 3 times 95% ethanol and stayed overnight at 4 °C. The precipitation was gathered by centrifugation, dissolved in distilled water, dialyzed in 4 °C distilled water for 2 d, digested with trypsin and precipitated protein with Sevag’s method. The polysaccharide extracts were dialyzed in 4 °C distilled water for another 2 d, precipitated with 3 times 95% ethanol, stayed overnight and collected with centrifuge. The white precipitation of polysaccharide extracts from the three edible fungi was rinsed with 100% ethanol and acetone and dried at room temperature.
The exponent growing SMMC-7721 cells in culture flasks were harvested by trypsinization with 0.25% trypsin, suspended in RPMI 1640 medium with 10% FCS, adjust ed to the concentration of 1 × 105 cells/mL, plated into 96-well plates (200 μL cells/well) and incubated at 37 °C in 5% CO2/95% air for 24 h. The medium was aspirated and the cells were washed with RPMI 1640 medium. The medium was replaced with RPMI 1640 containing 50 mg/L polysaccharide extracts from different fungi as treatment groups respectively and medium with 10% FCS and Non-FCS as controls (each group having 8 repeated wells). The cells were incubated in different treatments for 20 h, 44 h and 68 h respectively. The metabolism of mitochondria[4] were detected by adding 20 μL- MTT (final concentration 10 mg/L) to media, incubating for 4 h, sucking out the media, adding 100 μL dimethylsulfoxide (DMSO) to dissolve the violet-crystal and measuring the absorption at 570 nm.
The exponent growing SMMC-7721 cells were suspended in RPMI 1640 medium plus 10% FCS, adjusted to 1 × 105 cells/mL, plated 2 mL cells/well into 24-well plates (with cover glass, 4 repeated wells in each group) and incubated at 37 °C for 24 h. After incubated in medium containing different polysaccharide extracts (experiment groups) and 10% FCS or non-FCS (as control groups) for 24 h, the cells were fixed with Carnoy solution and stained with Feulgen reaction. The mitosis indexes were detected randomly by counting 1000 cells[5].
The SMMC-7721 cells were treated with different polysaccharide extracts for 7 d as above (6 repeated wells in each group) and the proliferation of SMMC-7721 cells were observed by counting the cell number every day.
The S-180 tumor cells were washed with normal physiological saline for three times, adjusted to 1 × 107 cells/mL and implanted by subcutaneous injection 200 μL to each mouse. Twenty-four h late, the mice were injectedip with 24 mg polysaccharide extracts/kg body weight in experimental groups and physiological saline in control group for 9 d. On the 15th day after the treatment, the mice were killed and the tumors were isolated and weighed. The inhibition rate of tumor was calculated as follows: (Mean tumor weight in controls-mean tumor weight in experiments)/(Mean tumor weight in control group) ×100%
The production of the polysaccharide extracts extracted from the three kinds of edible fungi were 1.53 mg/g ± 0.11 mg/g fresh fruit bodies of LE; 4 mg/g ± 0.15 mg/g fresh fruit bodies of FV and 1.3 mg/g ± 0.11 mg/g fresh fruit bodies of AB, respectively. None of the polysaccharide extracts showed obvious absorption in the range of 220 nm-780 nm.
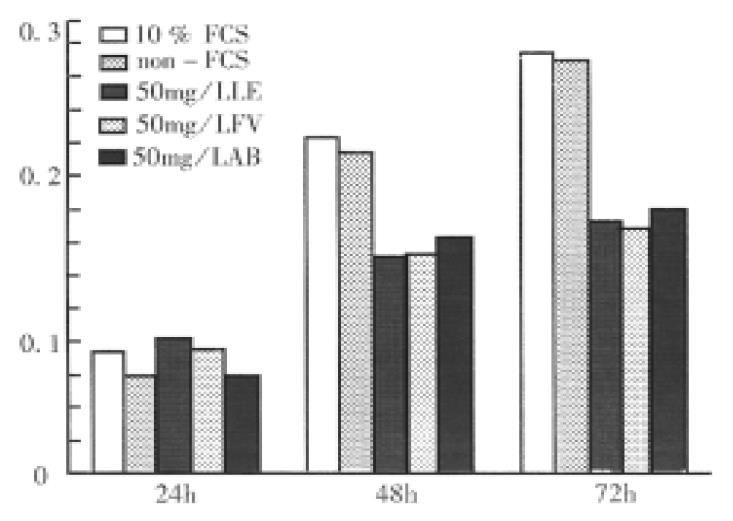
Within 24 h, the mitochondria metabolism of hepatoma SMMC-7721 cells showed no obvious differences in each group. At 48 h and 72 h, the metabolism activities in experimental groups were much lower than those in both FCS and non-FCS control groups, but in different experimental groups they were similar (Figure 1).
The MI in all the groups treated with polysaccharide extracts was lower than that in both control groups (P < 0.005) (Table 1).
| Groups | MI (%) | P value |
| C1 | 18.2 ± 1.1 | |
| C2 | 13.4 ± 1.2 | |
| E1 | 5.8 ± 0.7 | < 0.005 |
| E2 | 8.5 ± 0.9 | < 0.005 |
| E3 | 8.4 ± 0.8 | < 0.005 |
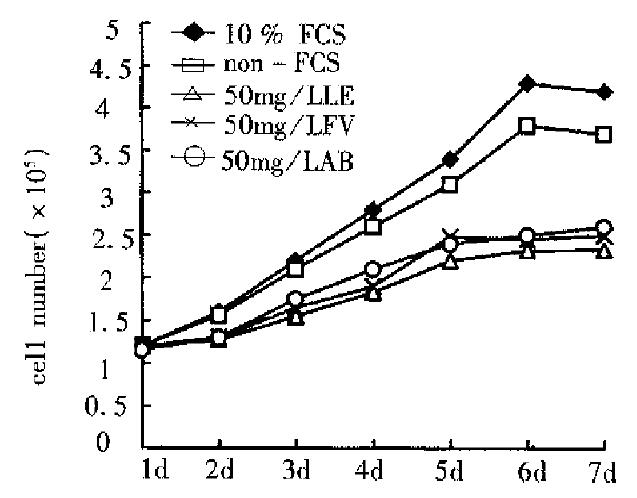
The cell number after treatment with polysaccharide extracts for 48 h were obviously lower as compared with both FCS and non-FCS controls (Figure 2). On the 7th day the number of the cells covered 55.5% (LE), 59.5% (FV) and 61.6% (AB) of that in the FCS control and 63% (LE), 67.6% (FV) and 70. 3% (AB) of that in non-FCS control.
The inhibitions of the three polysaccharide extracts on the proliferation of the S-180 tumor in vivo were 52.8% (LE), 56.6% (FV) and 51.9% (AB), respectively (Table 2).
| Groups | n | Dose (mg/kg) | Tumor weight (g) | Inhibition (%) | P value | Mean inhibition (%) |
| LE 1 | 10 | 24 | 0.369 ± 0.058 | 55.5 | < 0.005 | 52.8 |
| LE 2 | 10 | 24 | 0.387 ± 0.069 | 53.3 | < 0.005 | |
| LE 3 | 10 | 24 | 0.417 ± 0.085 | 49.7 | < 0.005 | |
| FV 1 | 10 | 24 | 0.371 ± 0.034 | 55.3 | < 0.005 | 56.6 |
| FV 2 | 10 | 24 | 0.348 ± 0.039 | 58.1 | < 0.005 | |
| FV 3 | 10 | 24 | 0.376 ± 0.044 | 54.7 | < 0.005 | |
| AB 1 | 10 | 24 | 0.379 ± 0.049 | 54.3 | < 0.005 | 51.9 |
| AB 2 | 10 | 24 | 0.39 ± 0.067 | 53 | < 0.005 | |
| AB 3 | 10 | 24 | 0.427 ± 0.087 | 48.5 | < 0.005 | |
| Control | 20 | 0.83 ± 0.17 |
Following the same processes, the products of polysaccharide extracts in the three kinds of edible fungi were obviously different. The product of polysaccharide extracts in FV was 4 mg/g fresh fruit body, which was 4 times higher than that in AB and 2.6 times than in LE. These differences should be induced by the contents of polysaccharide in different kinds of edible fungi. Although the polysaccharide extracts were a mixture of polysaccharides, they had no obvious absorption peak in the range of 220 nm-780 nm.
The polysaccharide from LE was the first extracts which verified the anti-tumor activity in 1969[1], which initiated the study of extraction, purification, structure analysis and anti-tumor activity of polysaccharides from LE. The best result of anti-tumor activity of polysaccharides from LE could reach 90%-100% in vivo. So polysaccharides from LE were believed to be one of the best effective substances for antitumor treatment. On the other hand the polysaccharide from LE was also verified to have anti-mutant activity[6]. Both pure and mixed polysaccharides from FV were shown to have strong anti-tumor activity against implanted S-180 tumor in mice[3,7]. In vivo the anti-tumor activities of polysaccharides from edible fungi were mainly induced by activating the immune system, although the detailed mechanism was not clear. In this study, the cultured hepatoma SMMC-7721 cells were used as a model to detect the activity of polysaccharide extracts in vitro. The results of MI, mitochondria activity and cell proliferation showed that the extracts from the edible fungi could inhibit the division of the cells and induce the decrease of cell proliferation, which was similar to that from fruit bodies of Ganoderma lucidum[8]. The inhibition of the extracts from the three kinds of edible fungi on the growth of implanted S-180 tumor in vivo could reach 52.8%, 56.6% and 51.9% respectively, which agreed with the previous reports[3].
These results suggested that the polysaccharide extracts from LE, FV and AB could not only inhibit the growth of implanted S-180 tumor in vivo but interfere with the proliferation of human hepatoma SMMC-7721 cells in vitro .
Edited by Ma JY
| 1. | Du YY. The advantage in the study of polysaccharide from Lentinus edodes. China Edible Fungi. 1995;14:9-11. |
| 2. | Guo JM, Shangguan ZJ, Chen JC. The study and usage of medicinal fungi. China Edible Fungi. 1994;13:8-10. |
| 3. | Chao PY, Wu ZD, Wang RC. The extraction, purification and analysis of polysaccharide PA3DE from the fruit body of Flammulina velutipes(Curt. ex Fr.) Sing. Acta Biochemica and Biophysica Sinica. 1989;21:152-156. |
| 4. | Huang WD, Wang YF. The colorimetry method for fast detection of cell proliferation and senescence. Chemist Life. 1994;14:44-45. |
| 5. | Jiang SM, Xu ZH, Shi XM, Zhang Y. The survival and malignant phenotype changes of human hepatoma SMMC-7721 cells induced by -50 cryopreservation. China Natl J New Gastroenterol. 1997;3. |
| 6. | Zheng JX. The anti-mutant activity of polysaccharide from fruit body. Lentinus Edodes. China Edible Fungi. 1994;13:5-7. |
| 7. | Yoshioka Y, Sano T, Ikekawa T. Studies on antitumor polysaccharides of Flammulina velutipes (Curt. ex Fr.) Sing. I. Chem Pharm Bull (Tokyo). 1973;21:1772-1776. [PubMed] |
| 8. | Xu ZH, Jiang SM. The extracts of Ganoderma Lucidum on DNA synthesis of hepatoma SMMC-7721 cells. China Edible Fungi. 1996;15:34-35. |