Published online Aug 15, 1999. doi: 10.3748/wjg.v5.i4.345
Revised: December 3, 1998
Accepted: December 19, 1998
Published online: August 15, 1999
- Citation: Shen Q, Li QF, Deng YZ, Zhang JM, Zhang J. Cloning and expression of HLA-B7 gene. World J Gastroenterol 1999; 5(4): 345-348
- URL: https://www.wjgnet.com/1007-9327/full/v5/i4/345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i4.345
In recent years, it has been found that HLA-B7 plays an important role in the antigen presentation and the research of human tumor therapy. It was reported that if HLA-B7 gene was transfected into target cells, the expression of HLA-B7 gene could stimulate the immune system to recognize the target cells and induce immune response[1]. In order to study the HLA-B7 gene vaccine and its feasibility for tumor gene therapy, HLA-B7 cDNA was cloned and an eukaryotic expression vector (pCD-B7) carrying HLA-B7 cDNA was constructed. This constructor was transfected into NIH/3T3 cells and injected into the subcutaneous tissue of mice, the expression of HLA-B7 gene could be detected both in vitro and in vivo.
Materials
Cells HLA-B7+ lymphocytes were isolated from human peripheral blood.
Regents Taq DNA polymerase, Supertranscript kit, and RPMI-1640 are products of GIBCO BRL. Primers were synthesized by Sangon Co. T4 DNA ligase, CIP, X-Gal, IPTG, restriction enzyme, DNA marker, calf serum, medium, PHA and IL-2 are purchased from Promega Company.
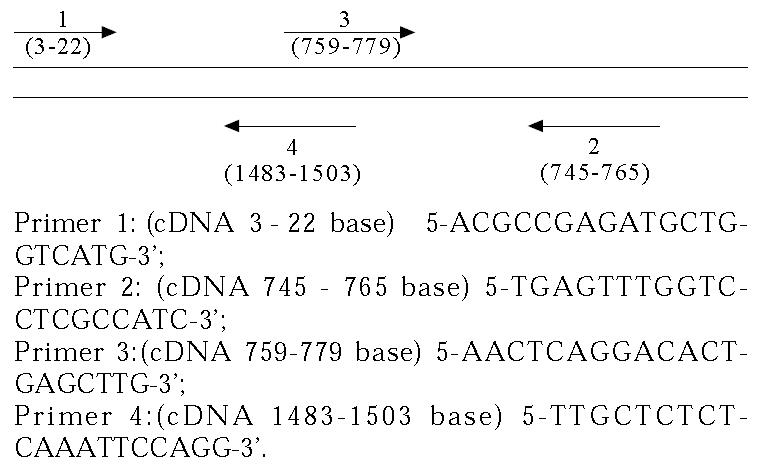
Primer designing The sites of the primers were chosen according to the cDNA sequence of HLA-B7 reported by Sood AK, et al[2]. Four primers were synthesized as follows:
Math 1
Selection and culture of HLA-B7+ lymphocytes Five mL peripheral blood was taken from each of 20 healthy persons. The lymphocytes were isolated and then counted. After cytotoxic test[3], the HLA-B7+ lymphocytes were cultured in medim RPMI 1640 containing 10% calf serum, PHA 50 mg/L and IL-2500 U/mL-1000 U/mL for 2-3 d.
Isolation of total RNA The total RNA was isolated by single step method[4]. The quantity and quality of RNA were analyzed by denaturing agarose gel.
RT-PCR Reverse transcription was performed according to the method recommended by GIBCO BRL supertranscription kit. Five μL products of reverse transcription was taken as templates for PCR. HL A-B7 target sequence was amplified for 35 cycles using prime 1, 2 and prime 3, 4 as well as prime 1, 4. Fifty μL reaction volume included 0.2 mmol/L each of dNTPs, 1.5 mmol/L MgCl2, 10 × 5 μL buffer, Taq DNA polymerase 2.5 U 0.25 mmol/L each of primer an d 5 μL sample. The reaction volume was denatured at 94 °C for 30 s, annealed at 53 °C for 1 min and extended at 72 °C for 2 min. After the last cycle of amplification, 10 μL of product was stained with ethidium bromide, and analyzed by agarosegel electrophoresis, and visualized under UV light.
Recombinant construction Ligation reaction was performed according to a rapid, easy and effective PCR product cloning method[5]. Forty μL PCR product, into which 1 μL (2.5 U) Klenow enzyme was added, was placed at 37 °C for 30 min and precipitated wit h ethanol. After drying, it was dissolved into 10 μL water. Three μL solution and 0.1 μg-pUC19 plasmid digested by Sma I and 15 μL- water were added into the tube of T4 DNA ligase, and kept at room temperature for 10 min. The ligated products were subjected to transform competent cells of HB101, and the positive clone was selected by α complementation detection.
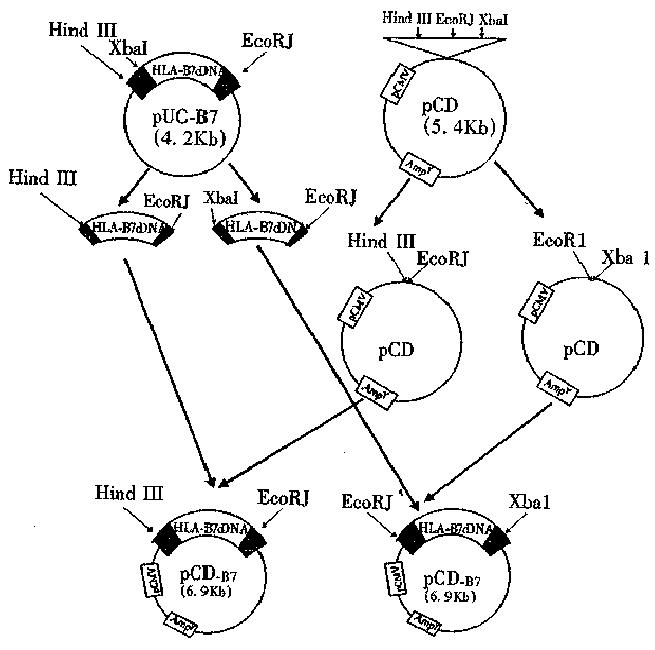
Construction of eukaryotic expression vector Construction procedure is shown in Figure 1. Before ligation, digested pcDNA3 was treated with CIP (calf intestinal alkaline phosphate) for 30 min. The treated linear vector was mixed, and then 2 μL 10 × buffer, 10 unit T4 DNA ligase, and TE (pH8.0) were added into HLA-B7 cDNA fragment (ratio 1:5) till the volume was up to 20 μL. The mixture was incubated at 16 °C for 16 h, and then at 70 °C for 10 min to terminate the reaction.
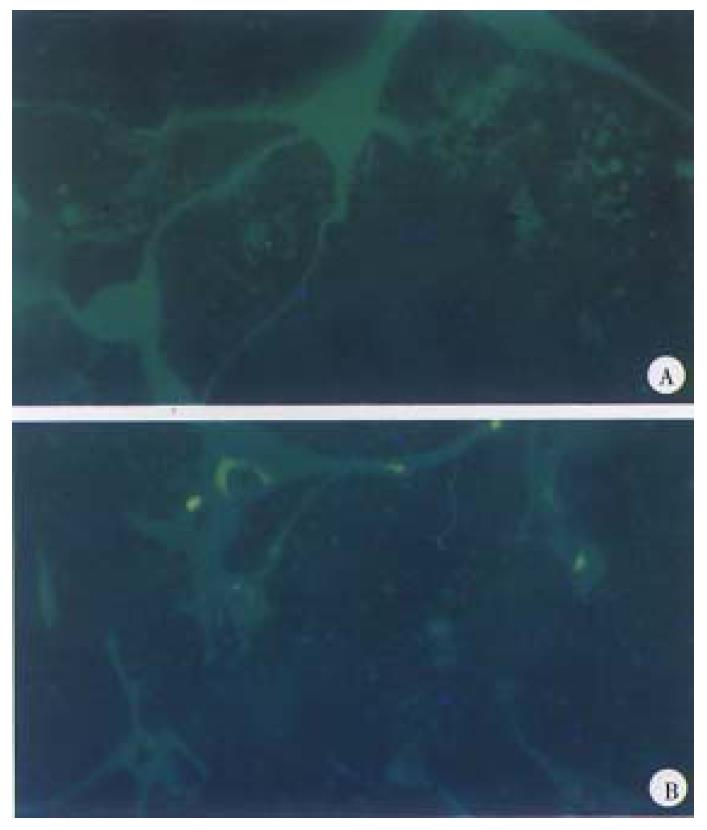
Transfection of pCD-B7 in vitro Lipofectin reagent was used for DNA transfection, logarithmically growing cells were seeded at 1 × 105 cells per 35 mm plate, and incubated overnight. Ten μg plasmid DNA and 9 μL lipofectin were diluted into 100 μL serum-free medium respectively, and allowed to stand at room temperature for 30-45 min. The two solutions were mixed and 0.8 mL serum-free medium was added and mixed gently and overlaid the complex onto cells. The cells were incubated for 24 h at 37 °C in a CO2 incubator. After the coverslips were taken out of the plate, the cells were fixed with cool actetone, and stored at -20 °C.
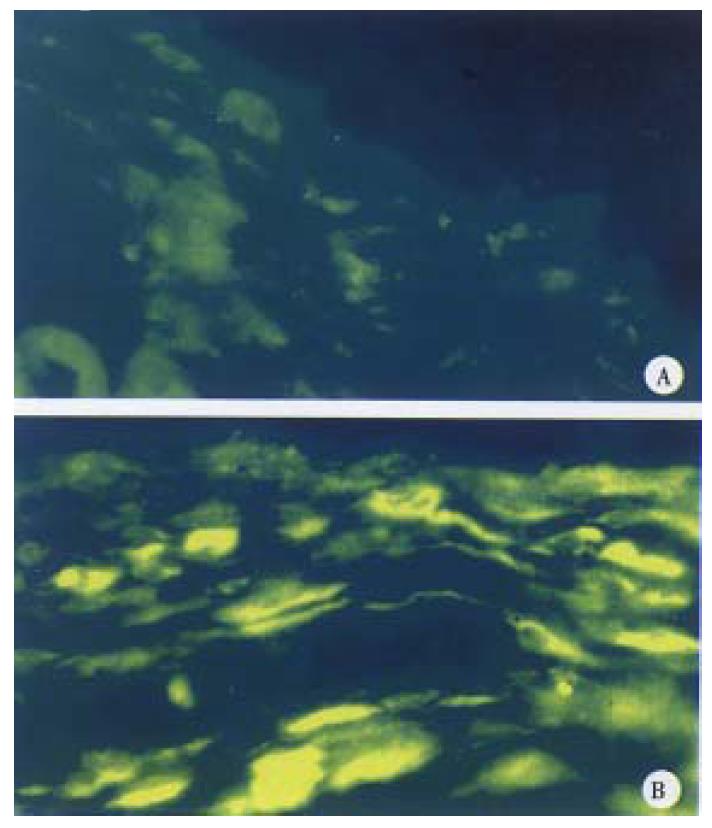
Injection of pCD-B7 in vivo The plasmid was dissolved in PBS (1 μg/μL). Forty μL of such plasmid solution was injected into the subcutaneou s layer on the back of mice. As a negative control, 40 μL- PBS was injected into the same tissue of mice. All injection points were marked. After 15 d, the animals were killed and the skin at the injection points were taken. The sections (10 μm) were frozen and fixed with cool actetone and stored at -20 °C.
Indirect fluorescent antibody technic was used to detect the expression of HL A-B7. HLA-B7 antibody (dilution 1:10) was added to the coverslip, incubated a t 4 °C overnight, washed with PBS for 2-3 times. Then FITC-goat antibody aga inst human IgG (dilution 1:20) was added and incubated at 37 °C for 30 min, washed with PBS 2-3 times and blocked with glycerol buffer. Finally the expression of HLA-B7 was observed under fluorescent microscopy.
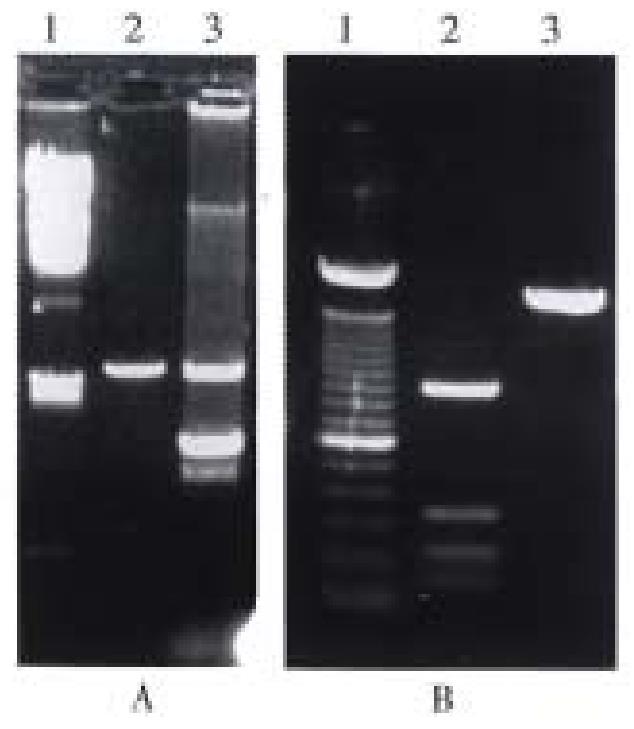
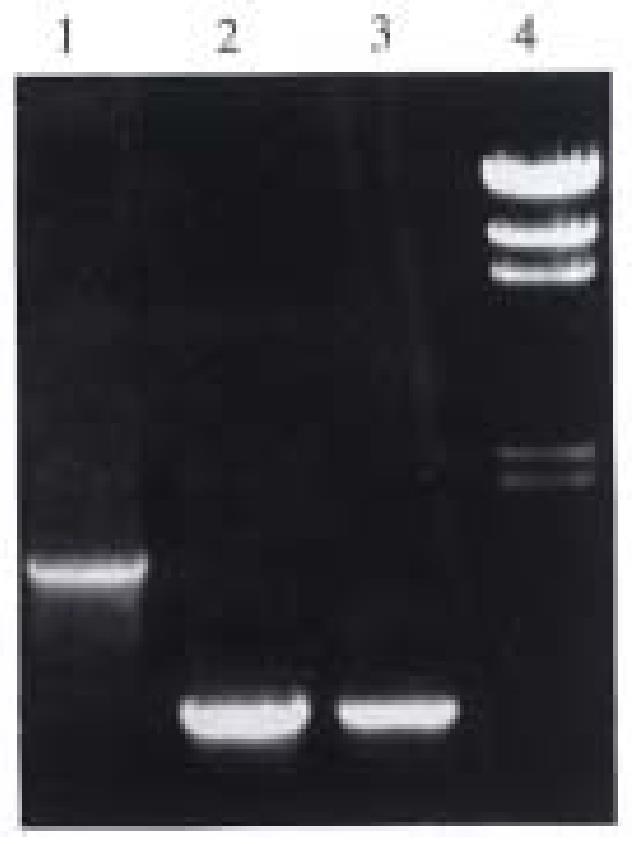
Identification of recombinant plasmid by restriction enzyme analysis The white bacterial colony was transferred into LB medium containing ampicillin. Then the plasmid DNA was prepared[6]. The pUC-B7 recombinant was digested with Hind III and EcoR I. The large fragment was 6.8 kb (pUC 19) and the small fragment was 1.5 kb (insert fragment). The small fragment was digested with Hinf I again. Four fragments could be seen on the running gel, their length was 976 bp, 230 bp and 200 bp, and 115 bp respectively and was consistent with the restriction sites of HLA-B 7 cDNA (Figure 3).
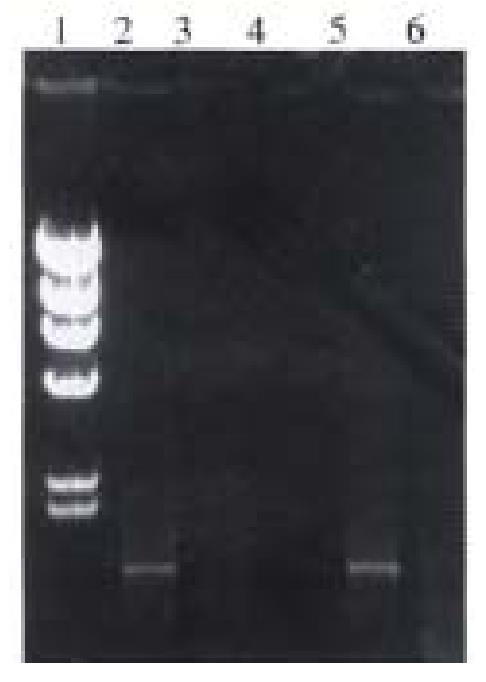
Identification of pUC-B7 by PCR The reaction condition of PCR has been discussed before 0.1 μL (0.1 μg) pUC-B7 was taken as template. The products of amplification including the full length, the upstream and the downstream region, showed no difference with those of the original products of PCR. So we could conclude that the insert in recombinant is identical to the products of original PCR (Figure 4).
The Amp + clone was selected and cultured in LB medium containing ampicillin. Plasmid DNA was prepared with small-scale alkaline lysis, and digested with Hind III and EcoR I. The length of the two fragments were 1.5 kb (HLA-B7 insertor) and 5.4 kb(vector) respectively. Four positive clonies were obtained.
Effective expression was achieved in two of the four positive clones, and was consistent with what we anticipated (Figure 5, Figure 6).
HLA-B7 cDNA was cloned by RT-PCR from human peripheral blood lymphocytes. The cDNA fragment was inserted into Sma I site of pUC19, most of the enzyme sites were kept for further construction. The pUC-B7 clone was identified by restriction enzyme digestion and PCR detection. The results corresponded to the data from Sood AK[2]. Furthermore, HLA-B7 cDNA fragment was cloned into pCDNA3. A eukaryotic expression vector was constructed and named pCD-B7. The result of indirect immuno- fluorescence technic demonstrated effective expression of HLA-B7 gene both in vitro and in vivo. This work provided the basis for further research on anti-tumor effect of HLA-B7 gene.
The study of tumor immune mechanism is deepening with the development of immunological theory. Two signal theory[7] accepted publicly laid a foundation for tumor immunotherapy. Activation of T lymphocyte requires two signals, one is the crosslinking of TCR by peptide-MHC complex, the other is the interaction between costimulatory molecule B7 and CD28 on the surface of T lymphocyte. HLA-B7 is one of the powerful signal molecules. There was a foreign report about treatment of HLA-B7 negative patient with melanoma by direct transfer of HLA-B7 gene in vivo[1]. The efficacy is positive. Tumor cell transfected with HLA-B7 could not grow in vivo[8]. It acts as a vaccine to induce immune response, so that unmodified tumor cells could be recognized and destroyed by immune system. The study demonstrated that direct transfer of HLA-B7 gene to human in vivo is safe, feasible and potent. If HLA-B7 gene can be made into DNA vaccine with corresponding efficacy in vivo, it will be a hopeful way for tumor gene therapy.
Qu Shen, male, born on 1954-08-13 in Wuhan, Hubei Province, China and graduated from Tongji Medical University, professor of biochemistry, specializing in research of gene diagnosis and gene therapy, having 35 papers published.
Edited by WANG Xian-Lin
| 1. | Nabel GJ, Nabel EG, Yang ZY, Fox BA, Plautz GE, Gao X, Huang L, Shu S, Gordon D, Chang AE. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci USA. 1993;90:11307-11311. [PubMed] |
| 2. | Sood AK, Pan J, Biro PA, Pereira D, Srivastava R, Reddy VB, Duceman BW, Weissman SM. Structure and polymorphism of class I MHC antigen mRNA. Immunogenetics. 1985;22:101-121. [PubMed] |
| 3. | Zhao TM. HLA typing principles. Beijing: People′s Health Publishing House 1988; 412-413. |
| 4. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] |
| 5. | Li HW, Tu CC, Jin KS, Zhang JG. A rapid, simple and effective clone method for PCR products. Biochemistry. 1995;15:28-30. |
| 6. | Lu SD. Laboratory techniques of modern molecular biology. Beijing: Higher Education Publishing House 1993; 412-413. |
| 7. | Travis J. A stimulating new approach to cancer treatment. Science. 1993;259:310-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Leong CC, Robinson BW, Garlepp MJ. Generation of an antitumour immune response to a murine mesothelioma cell line by the transfection of allogeneic MHC genes. Int J Cancer. 1994;59:212-216. [PubMed] |